One-Year Outcome Trajectories and Factors Associated with Functional Recovery Among Survivors of Intracerebral and Intraventricular Hemorrhage With Initial Severe Disability
- PMID: 35877105
- PMCID: PMC9316056
- DOI: 10.1001/jamaneurol.2022.1991
One-Year Outcome Trajectories and Factors Associated with Functional Recovery Among Survivors of Intracerebral and Intraventricular Hemorrhage With Initial Severe Disability
Abstract
Importance: Patients who survive severe intracerebral hemorrhage (ICH) and intraventricular hemorrhage (IVH) typically have poor functional outcome in the short term and understanding of future recovery is limited.
Objective: To describe 1-year recovery trajectories among ICH and IVH survivors with initial severe disability and assess the association of hospital events with long-term recovery.
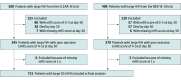
Design, setting, and participants: This post hoc analysis pooled all individual patient data from the Clot Lysis: Evaluating Accelerated Resolution of Intraventricular Hemorrhage phase 3 trial (CLEAR-III) and the Minimally Invasive Surgery Plus Alteplase for Intracerebral Hemorrhage Evacuation (MISTIE-III) phase 3 trial in multiple centers across the US, Canada, Europe, and Asia. Patients were enrolled from August 1, 2010, to September 30, 2018, with a follow-up duration of 1 year. Of 999 enrolled patients, 724 survived with a day 30 modified Rankin Scale score (mRS) of 4 to 5 after excluding 13 participants with missing day 30 mRS. An additional 9 patients were excluded because of missing 1-year mRS. The final pooled cohort included 715 patients (71.6%) with day 30 mRS 4 to 5. Data were analyzed from July 2019 to January 2022.
Exposures: CLEAR-III participants randomized to intraventricular alteplase vs placebo. MISTIE-III participants randomized to stereotactic thrombolysis of hematoma vs standard medical care.
Main outcomes and measures: Primary outcome was 1-year mRS. Patients were dichotomized into good outcome at 1 year (mRS 0 to 3) vs poor outcome at 1 year (mRS 4 to 6). Multivariable logistic regression models assessed associations between prospectively adjudicated hospital events and 1-year good outcome after adjusting for demographic characteristics, ICH and IVH severity, and trial cohort.
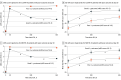
Results: Of 715 survivors, 417 (58%) were male, and the overall mean (SD) age was 60.3 (11.7) years. Overall, 174 participants (24.3%) were Black, 491 (68.6%) were White, and 49 (6.9%) were of other races (including Asian, Native American, and Pacific Islander, consolidated owing to small numbers); 98 (13.7%) were of Hispanic ethnicity. By 1 year, 129 participants (18%) had died and 308 (43%) had achieved mRS 0 to 3. In adjusted models for the combined cohort, diabetes (adjusted odds ratio [aOR], 0.50; 95% CI, 0.26-0.96), National Institutes of Health Stroke Scale (aOR, 0.93; 95% CI, 0.90-0.96), severe leukoaraiosis (aOR, 0.30; 95% CI, 0.16-0.54), pineal gland shift (aOR, 0.87; 95% CI, 0.76-0.99]), acute ischemic stroke (aOR, 0.44; 95% CI, 0.21-0.94), gastrostomy (aOR, 0.30; 95% CI, 0.17-0.50), and persistent hydrocephalus by day 30 (aOR, 0.37; 95% CI, 0.14-0.98) were associated with lack of recovery. Resolution of ICH (aOR, 1.82; 95% CI, 1.08-3.04) and IVH (aOR, 2.19; 95% CI, 1.02-4.68) by day 30 were associated with recovery to good outcome. In the CLEAR-III model, cerebral perfusion pressure less than 60 mm Hg (aOR, 0.30; 95% CI, 0.13-0.71), sepsis (aOR, 0.05; 95% CI, 0.00-0.80), and prolonged mechanical ventilation (aOR, 0.96; 95% CI, 0.92-1.00 per day), and in MISTIE-III, need for intracranial pressure monitoring (aOR, 0.35; 95% CI, 0.12-0.98), were additional factors associated with poor outcome. Thirty-day event-based models strongly predicted 1-year outcome (area under the receiver operating characteristic curve [AUC], 0.87; 95% CI, 0.83-0.90), with significantly improved discrimination over models using baseline severity factors alone (AUC, 0.76; 95% CI, 0.71-0.80; P < .001).
Conclusions and relevance: Among survivors of severe ICH and IVH with initial poor functional outcome, more than 40% recovered to good outcome by 1 year. Hospital events were strongly associated with long-term functional recovery and may be potential targets for intervention. Avoiding early pessimistic prognostication and delaying prognostication until after treatment may improve ability to predict future recovery.
Conflict of interest statement
Figures

Comment in
-
Time for a New Perspective on Intracerebral Hemorrhage.JAMA Neurol. 2022 Sep 1;79(9):844-845. doi: 10.1001/jamaneurol.2022.1988. JAMA Neurol. 2022. PMID: 35877098 No abstract available.
References
-
- van Asch CJ, Luitse MJ, Rinkel GJ, van der Tweel I, Algra A, Klijn CJM. Incidence, case fatality, and functional outcome of intracerebral haemorrhage over time, according to age, sex, and ethnic origin: a systematic review and meta-analysis. Lancet Neurol. 2010;9(2):167-176. doi:10.1016/S1474-4422(09)70340-0 - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

