Cerebral vascular injury in transplant-associated thrombotic microangiopathy
- PMID: 35877136
- PMCID: PMC9327538
- DOI: 10.1182/bloodadvances.2022007453
Cerebral vascular injury in transplant-associated thrombotic microangiopathy
Abstract
Transplant-associated thrombotic microangiopathy (TA-TMA) and atypical hemolytic uremic syndrome (aHUS) are complement-mediated TMAs. The central nervous system (CNS) is the most common extrarenal organ affected by aHUS, and, despite mechanistic overlap between aHUS and TA-TMA, CNS involvement is rarely reported in TA-TMA, suggesting that CNS involvement in TA-TMA may be underdiagnosed and that these patients may benefit from complement blockers. In addition, there are no widely used histologic or radiologic criteria for the diagnosis of TMA in the brain. Thirteen recipients of pediatric hematopoietic cell transplants (HCTs) who had TA-TMA and who underwent autopsy were studied. Seven of 13 brains had vascular injury, and 2 had severe vascular injury. Neurologic symptoms correlated with severe vascular injury. Classic TMA histology was present and most often observed in the cerebellum, brainstem, and cerebral white matter. Abnormalities in similar anatomic regions were seen on imaging. Brain imaging findings related to TMA included hemorrhages, siderosis, and posterior reversible encephalopathy syndrome. We then studied 100 consecutive HCT recipients to identify differences in neurologic complications between patients with and those without TA-TMA. Patients with TA-TMA were significantly more likely to have a clinical concern for seizure, have an electroencephalogram performed, and develop altered mental status. In summary, our study confirms that TA-TMA involves the brains of recipients of HCT and is associated with an increased incidence of neurologic symptoms. Based on these findings, we propose that patients with low- or moderate-risk TA-TMA who develop neurologic complications should be considered for TA-TMA-directed therapy.
© 2022 by The American Society of Hematology. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Figures
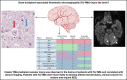

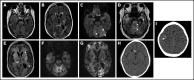

References
-
- Jodele S, Sabulski A. Transplant-associated thrombotic microangiopathy: elucidating prevention strategies and identifying high-risk patients. Expert Rev Hematol. 2021;14(8):751-763. - PubMed
-
- Riedl M, Fakhouri F, Le Quintrec M, et al. . Spectrum of complement-mediated thrombotic microangiopathies: pathogenetic insights identifying novel treatment approaches. Semin Thromb Hemost. 2014;40(4):444-464. - PubMed
-
- Noris M, Remuzzi G. Cardiovascular complications in atypical haemolytic uraemic syndrome. Nat Rev Nephrol. 2014;10(3):174-180. - PubMed

