Real-World Clinical Outcomes after Genomic Profiling of Circulating Tumor DNA in Patients with Previously Treated Advanced Non-Small Cell Lung Cancer
- PMID: 35877242
- PMCID: PMC9318660
- DOI: 10.3390/curroncol29070382
Real-World Clinical Outcomes after Genomic Profiling of Circulating Tumor DNA in Patients with Previously Treated Advanced Non-Small Cell Lung Cancer
Abstract
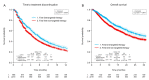
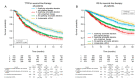
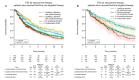
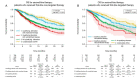
Comprehensive genomic profiling for advanced non-small cell lung cancer (NSCLC) can identify patients for molecularly targeted therapies that improve clinical outcomes. We analyzed data from 3084 patients (median age 65 years, 72.9% with adenocarcinoma) with advanced NSCLC registered in a real-world healthcare claims database (GuardantINFORMTM, Guardant Health) who underwent next-generation sequencing (NGS)-based circulating tumor DNA (ctDNA) testing (Guardant360®, Guardant Health) after first-line therapy (28.0% with agents targeted against genomic alterations). ctDNA was detected in 2771 samples (89.9%), of which 41.9% harbored actionable alterations, most commonly EGFR (epidermal growth factor receptor) mutations (29.7%). Actionable alterations were detected in 26.7% of patients (534/2001) previously treated with non-targeted agents. Emerging potentially targetable mutations were found in 40.1% (309/770) of patients previously treated with targeted therapies. Among patients with qualifying alterations detected by ctDNA testing, the time to treatment discontinuation (median 8.8 vs. 4.2 months; hazard ratio 1.97, p < 0.001) and overall survival (median 36.1 vs. 16.6 months; hazard ratio 2.08, p < 0.001) were longer for those who received matched second-line therapy versus unmatched second-line therapy. In real-world practice, results of a blood-based NGS assay prior to second-line treatment inform therapeutic decisions that can improve clinical outcomes for patients with advanced NSCLC.
Keywords: actionable alterations; comprehensive genomic profiling; ctDNA; non-small cell lung cancer; targeted therapy.
Conflict of interest statement
S.O. is an employee of Guardant Health, Inc., and holds stock in Guardant Health, Inc and AstraZeneca. J.L. is an employee of Guardant Health, Inc. H.H. reports grants from AstraZeneca K.K., Bristol-Myers Squibb Co. Ltd. Boehringer Ingelheim Japan Inc., Bristol-Myers Squibb Co. Ltd., Chugai Pharmaceutical Co. Ltd., and Ono Pharmaceutical Co. Ltd. and personal fees from AstraZeneca K.K., Boehringer Ingelheim Japan Inc., Bristol-Myers Squibb Co. Ltd., Chugai Pharmaceutical Co. Ltd., Daiichi Sankyo Co. Ltd., Eli Lilly Japan K.K., Guardant Health, Inc., Kyorin Pharmaceutical Co. Ltd., Merck Biopharma Co. Ltd., MSD K.K., Novartis Pharmaceuticals K.K., Ono Pharmaceutical Co. Ltd., Shanghai Haihe Biopharm, Taiho Pharmaceutical Co. Ltd., Pfizer, and Takeda Pharmaceutical Co. Ltd.
Figures
References
-
- Hanna N.H., Robinson A.G., Temin S., Baker S., Jr., Brahmer J.R., Ellis P.M., Gaspar L.E., Haddad R.Y., Hesketh P.J., Jain D., et al. Therapy for Stage IV Non–Small-Cell Lung Cancer With Driver Alterations: ASCO and OH (CCO) Joint Guideline Update. J. Clin. Oncol. 2021;39:1040–1091. doi: 10.1200/JCO.20.03570. - DOI - PubMed
-
- Lindeman N.I., Cagle P.T., Aisner D.L., Arcila M.E., Beasley M.B., Bernicker E.H., Colasacco C., Dacic S., Hirsch F.R., Kerr K., et al. Updated Molecular Testing Guideline for the Selection of Lung Cancer Patients for Treatment With Targeted Tyrosine Kinase Inhibitors: Guideline From the College of American Pathologists, the International Association for the Study of Lung Cancer, and the Association for Molecular Pathology. J. Thorac. Oncol. 2018;13:323–358. doi: 10.1016/j.jtho.2017.12.001. - DOI - PubMed
-
- National Comprehensive Cancer Network NCCN Clinical Practice Guidelines in Oncology. Non-small Cell Lung Cancer Version 1.2022—7 December 2021. [(accessed on 6 April 2022)]. Available online: https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf.
-
- Planchard D., Popat S., Kerr K., Novello S., Smit E.F., Faivre-Finn C., Mok T.S., Reck M., Van Schil P.E., Hellmann M.D., et al. Metastatic non-small cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2018;29:iv192–iv237. doi: 10.1093/annonc/mdy275. Correction in Ann. Oncol. 2019, 2030, 2863–2070. - DOI - PubMed
-
- Rolfo C., Mack P., Scagliotti G.V., Aggarwal C., Arcila M.E., Barlesi F., Bivona T., Diehn M., Dive C., Dziadziuszko R., et al. Liquid Biopsy for Advanced NSCLC: A Consensus Statement From the International Association for the Study of Lung Cancer. J. Thorac. Oncol. 2021;16:1647–1662. doi: 10.1016/j.jtho.2021.06.017. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

