Appraising Adjuvant Endocrine Therapy in Hormone Receptor Positive HER2-Negative Breast Cancer-A Literature Review
- PMID: 35877254
- PMCID: PMC9320044
- DOI: 10.3390/curroncol29070394
Appraising Adjuvant Endocrine Therapy in Hormone Receptor Positive HER2-Negative Breast Cancer-A Literature Review
Abstract
Background: Approximately 75% of breast cancer (BC) is associated with luminal differentiation expressing endocrine receptors (ER). For ER+ HER2- tumors, adjuvant endocrine therapy (ET) is the cornerstone treatment. Although relapse events steadily continue, the ET benefits translate to dramatically lengthen life expectancy with bearable side-effects. This review of ER+ HER2- female BC outlines suitable adjuvant treatment strategies to help guide clinical decision making around appropriate therapy.
Methods: A literature search was conducted in Embase, Medline, and the Cochrane Libraries, using ER+ HER-, ET BC keywords.
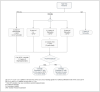
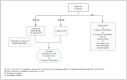
Results: In low-risk patients: five years of ET is the standard option. While Tamoxifen remains the preferred selection for premenopausal women, AI is the choice for postmenopausal patients. In the high-risk category: ET plus/minus OFS with two years of Abemaciclib is recommended. Although extended ET for a total of ten years is an alternative, the optimal AI duration is undetermined; nevertheless an additional two to three years beyond the initial five years may be sufficient. In this postmenopausal group, bisphosphonate is endorsed.
Conclusions: Classifying the risk category assists in deciding the treatment route and its optimal duration. Tailoring the breadth of ET hinges on a wide array of factors to be appraised for each individualized case, including weighing its benefits and harms.
Keywords: HER2-negative; adjuvant cyclin-dependent kinases 4/6 inhibitors; aromatase inhibitors; bisphosphonates; early breast cancer; endocrine therapy; hormone receptor positive; postmenopausal; premenopausal; selective estrogen receptor.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Davies C., Godwin J., Gray R., Clarke M., Darby S., McGale P., Wang Y.C., Peto R., Pan H.C., Cutter D., et al. Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: Patient-level meta-analysis of randomised trials. Lancet. 2011;378:771–784. doi: 10.1016/s0140-6736(11)60993-8. - DOI - PMC - PubMed
-
- Evidence Reviews for Adjuvant Systemic Therapy Planning: Early and Locally Advanced Breast Cancer: Diagnosis and Management. [(accessed on 22 June 2022)]; Available online: https://pubmed.ncbi.nlm.nih.gov/35073003/ - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous