Anti-EpCAM Functionalized I-131 Radiolabeled Biomimetic Nanocarrier Sodium/Iodide-Symporter-Mediated Breast-Cancer Treatment
- PMID: 35877345
- PMCID: PMC9311516
- DOI: 10.3390/bioengineering9070294
Anti-EpCAM Functionalized I-131 Radiolabeled Biomimetic Nanocarrier Sodium/Iodide-Symporter-Mediated Breast-Cancer Treatment
Abstract
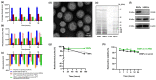
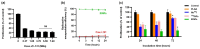
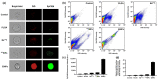
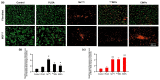
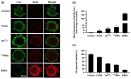
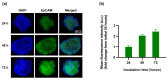
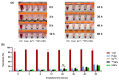
Currently, breast-cancer treatment has a number of adverse side effects and is associated with poor rates of progression-free survival. Therefore, a radiolabeled anti-EpCAM targeted biomimetic coated nanocarrier (EINP) was developed in this study to overcome some of the treatment challenges. The double emulsion method synthesized the poly(lactic-co-glycolic acid) (PLGA) nanoparticle with Na131I entrapped in the core. The PLGA nanoparticle was coated in human red blood cell membranes and labeled with epithelial cell adhesion molecule (EpCAM) antibody to enable it to target EpCAM overexpression by breast-cancer cells. Characterization determined the EINP size as 295 nm, zeta potential as −35.9 mV, and polydispersity as 0.297. EINP radiochemical purity was >95%. Results determined the EINP efficacy against EpCAM positive MCF-7 breast cancer at 24, 48, and 72 h were 69.11%, 77.84%, and 74.6%, respectively, demonstrating that the EINPs achieved greater cytotoxic efficacy supported by NIS-mediated Na131I uptake than the non-targeted 131INPs and Na131I. In comparison, fibroblast (EpCAM negative) treated with EINPs had significantly lower cytotoxicity than Na131I and 131INPs (p < 0.05). Flow cytometry fluorescence imaging visually signified delivery by EINPs specifically to breast-cancer cells as a result of anti-EpCAM targeting. Additionally, the EINP had a favorable safety profile, as determined by hemolysis.
Keywords: EpCAM; I-131; PLGA; breast cancer; drug delivery; ionizing radiation; nanoparticle.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Anti-EGFR Targeted Multifunctional I-131 Radio-Nanotherapeutic for Treating Osteosarcoma: In Vitro 3D Tumor Spheroid Model.Nanomaterials (Basel). 2022 Oct 8;12(19):3517. doi: 10.3390/nano12193517. Nanomaterials (Basel). 2022. PMID: 36234645 Free PMC article.
-
Evaluation of Single Dose and Fractionated Dose of I-131 Radiolabeled Nanoparticles for Triple-Negative Breast Cancer Treatment.Biomedicines. 2023 Aug 1;11(8):2169. doi: 10.3390/biomedicines11082169. Biomedicines. 2023. PMID: 37626666 Free PMC article.
-
Enhanced in vitro antiproliferative effects of EpCAM antibody-functionalized paclitaxel-loaded PLGA nanoparticles in retinoblastoma cells.Mol Vis. 2011;17:2724-37. Epub 2011 Oct 19. Mol Vis. 2011. Retraction in: Mol Vis. 2013 Jun 06;19:1258. PMID: 22065926 Free PMC article. Retracted.
-
Novel epithelial cell adhesion molecule antibody conjugated polyethyleneimine-capped gold nanoparticles for enhanced and targeted small interfering RNA delivery to retinoblastoma cells.Mol Vis. 2013 May 6;19:1029-38. Print 2013. Mol Vis. 2013. PMID: 23687439 Free PMC article.
-
Epithelial cell adhesion molecule aptamer conjugated PEG-PLGA nanopolymersomes for targeted delivery of doxorubicin to human breast adenocarcinoma cell line in vitro.Int J Pharm. 2015 Feb 1;479(1):241-51. doi: 10.1016/j.ijpharm.2014.12.035. Epub 2014 Dec 18. Int J Pharm. 2015. PMID: 25529433
Cited by
-
Anti-EGFR Targeted Multifunctional I-131 Radio-Nanotherapeutic for Treating Osteosarcoma: In Vitro 3D Tumor Spheroid Model.Nanomaterials (Basel). 2022 Oct 8;12(19):3517. doi: 10.3390/nano12193517. Nanomaterials (Basel). 2022. PMID: 36234645 Free PMC article.
-
Evaluation of Single Dose and Fractionated Dose of I-131 Radiolabeled Nanoparticles for Triple-Negative Breast Cancer Treatment.Biomedicines. 2023 Aug 1;11(8):2169. doi: 10.3390/biomedicines11082169. Biomedicines. 2023. PMID: 37626666 Free PMC article.
References
-
- Runowicz C.D., Leach C.R., Henry N.L., Henry K.S., Mackey H.T., Cowens-Alvarado R.L., Cannady R.S., Pratt-Chapman M.L., Edge S.B., Jacobs L.A. American Cancer Society/American Society of Clinical Oncology Breast Cancer Survivorship Care Guideline. CA Cancer J. Clin. 2016;66:43–73. doi: 10.3322/caac.21319. - DOI - PubMed
-
- Brito A.F., Abrantes A.M., Teixo R., Pires A.S., Ribeiro A.C., Ferreira R.F., Mascarenhas A., Puga T., Laranjo M., Caramelo F. Iodine-131 Metabolic Radiotherapy Leads to Cell Death and Genomic Alterations through NIS Overexpression on Cholangiocarcinoma. Int. J. Oncol. 2020;56:709–727. doi: 10.3892/ijo.2020.4957. - DOI - PMC - PubMed
-
- Spitzweg C., Zhang S., Bergert E.R., Castro M.R., McIver B., Heufelder A.E., Tindall D.J., Young C.Y., Morris J.C. Prostate-Specific Antigen (PSA) Promoter-Driven Androgen-Inducible Expression of Sodium Iodide Symporter in Prostate Cancer Cell Lines. Cancer Res. 1999;59:2136–2141. - PubMed
-
- Huang M., Batra R.K., Kogai T., Lin Y.Q., Hershman J.M., Lichtenstein A., Sharma S., Zhu L.X., Brent G.A., Dubinett S.M. Ectopic Expression of the Thyroperoxidase Gene Augments Radioiodide Uptake and Retention Mediated by the Sodium Iodide Symporter in Non–Small Cell Lung Cancer. Cancer Gene Ther. 2001;8:612–618. doi: 10.1038/sj.cgt.7700354. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

