Microcarrier-Based Culture of Human Pluripotent Stem-Cell-Derived Retinal Pigmented Epithelium
- PMID: 35877348
- PMCID: PMC9311890
- DOI: 10.3390/bioengineering9070297
Microcarrier-Based Culture of Human Pluripotent Stem-Cell-Derived Retinal Pigmented Epithelium
Abstract
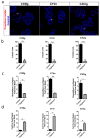
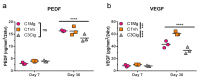
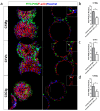
Dry age-related macular degeneration (AMD) is estimated to impact nearly 300 million individuals globally by 2040. While no treatment options are currently available, multiple clinical trials investigating retinal pigmented epithelial cells derived from human pluripotent stem cells (hPSC-RPE) as a cellular replacement therapeutic are currently underway. It has been estimated that a production capacity of >109 RPE cells annually would be required to treat the afflicted population, but current manufacturing protocols are limited, being labor-intensive and time-consuming. Microcarrier technology has enabled high-density propagation of many adherent mammalian cell types via monolayer culture on surfaces of uM-diameter matrix spheres; however, few studies have explored microcarrier-based culture of RPE cells. Here, we provide an approach to the growth, maturation, and differentiation of hPSC-RPE cells on Cytodex 1 (C1) and Cytodex 3 (C3) microcarriers. We demonstrate that hPSC-RPE cells adhere to microcarriers coated with Matrigel, vitronectin or collagen, and mature in vitro to exhibit characteristic epithelial cell morphology and pigmentation. Microcarrier-grown hPSC-RPE cells (mcRPE) are viable; metabolically active; express RPE signature genes including BEST1, RPE65, TYRP1, and PMEL17; secrete the trophic factors PEDF and VEGF; and demonstrate phagocytosis of photoreceptor outer segments. Furthermore, we show that undifferentiated hESCs also adhere to Matrigel-coated microcarriers and are amenable to directed RPE differentiation. The capacity to support hPSC-RPE cell cultures using microcarriers enables efficient large-scale production of therapeutic RPE cells sufficient to meet the treatment demands of a large AMD patient population.
Keywords: microcarriers; retinal pigment epithelium; stem cells.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Wong W.L., Su X., Li X., Cheung C.M.G., Klein R., Cheng C.Y., Wong T.Y. Global Prevalence of Age-Related Macular Degeneration and Disease Burden Projection for 2020 and 2040: A Systematic Review and Meta-Analysis. Lancet Glob. Health. 2014;2:e106–e116. doi: 10.1016/S2214-109X(13)70145-1. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

