Development of an In Vivo Model for Eustachian Tube Dysfunction
- PMID: 35877368
- PMCID: PMC9311709
- DOI: 10.3390/bioengineering9070317
Development of an In Vivo Model for Eustachian Tube Dysfunction
Abstract
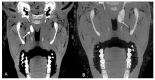
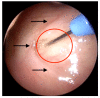
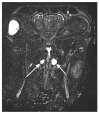
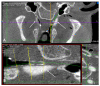
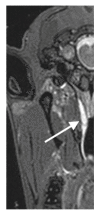
Otitis media is often connected to Eustachian tube dysfunction (ETD). Until now, there was no large animal model available for the examination of new treatment methods such as stents for the Eustachian tube (ET). Thus, the aim of the study was to develop a method to reproducibly induce ETD by injection of fillers and without permanent closure of the ET. Tools for safe injection of hyaluronic acid (HA) in the surrounding of the ET were developed. In ex vivo experiments, HA mixed with Imeron® was injected close to the nasopharyngeal orifice of the ET of blackface sheep. The established depot was visualized using cone beam computer tomography and magnetic resonance imaging, and stents could be placed into the ET. A reliable position of the HA depot was achieved. This method was transferred to in vivo, and middle ear ventilation was investigated by tympanometry. ETD was achieved with amounts of 2.5 mL HA or higher. None of the animals showed any sign of discomfort or complications. The induced ETD lasted for 3 to 13 (maximum observation period) weeks and was also combined with middle ear effusion. A model of ETD based on injection of HA next to the ET was successfully established and is now available to test novel treatment options for ET functionality.
Keywords: Eustachian tube dysfunction; animal model; cone beam CT; hyaluronic acid; otitis media with effusion; tympanometry.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

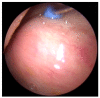
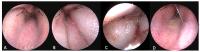
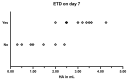
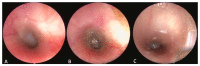
Similar articles
-
First Investigation of a Eustachian Tube Stent in Experimentally Induced Eustachian Tube Dysfunction.Bioengineering (Basel). 2024 Oct 11;11(10):1015. doi: 10.3390/bioengineering11101015. Bioengineering (Basel). 2024. PMID: 39451391 Free PMC article.
-
Stenting the Eustachian tube to treat chronic otitis media - a feasibility study in sheep.Head Face Med. 2018 May 4;14(1):8. doi: 10.1186/s13005-018-0165-5. Head Face Med. 2018. PMID: 29728102 Free PMC article.
-
Eustachian Tube Dysfunction in Children with Adenoid Hypertrophy: The Effect of Intranasal Azelastine-Fluticasone Spray Treatment on Middle Ear Ventilation and Adenoid Tissue.Ear Nose Throat J. 2023 Mar;102(3):198-203. doi: 10.1177/01455613221140281. Epub 2022 Nov 23. Ear Nose Throat J. 2023. PMID: 36416201
-
Allergy in pathogenesis of Eustachian Tube Dysfunction.World Allergy Organ J. 2024 Jan 5;17(1):100860. doi: 10.1016/j.waojou.2023.100860. eCollection 2024 Jan. World Allergy Organ J. 2024. PMID: 38274710 Free PMC article. Review.
-
[Progress in the tests of eustachian tube function].Lin Chuang Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2016 Jul 20;30(14):1171-1175. doi: 10.13201/j.issn.1001-1781.2016.14.023. Lin Chuang Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2016. PMID: 29798451 Review. Chinese.
Cited by
-
First Investigation of a Eustachian Tube Stent in Experimentally Induced Eustachian Tube Dysfunction.Bioengineering (Basel). 2024 Oct 11;11(10):1015. doi: 10.3390/bioengineering11101015. Bioengineering (Basel). 2024. PMID: 39451391 Free PMC article.
-
Intravascular Ultrasonography (IVUS)-A Tool for Imaging the Eustachian Tube?Bioengineering (Basel). 2022 Nov 28;9(12):733. doi: 10.3390/bioengineering9120733. Bioengineering (Basel). 2022. PMID: 36550939 Free PMC article.
-
Long-Term Preclinical Evaluation of a Permanent Stent Developed for the Human Eustachian Tube.Bioengineering (Basel). 2024 Jul 25;11(8):755. doi: 10.3390/bioengineering11080755. Bioengineering (Basel). 2024. PMID: 39199713 Free PMC article.
References
-
- Lapsley R.M. Diseases of the Middle Ear. Laryngoscope. 1898;5:170–172. doi: 10.1288/00005537-189809000-00015. - DOI
-
- Healy G.B., Rosbe K.W. Otitis Media and Middle Ear Effusions. In: Snow J.B., Ballenger J.J., editors. Ballenger’s Otorhinolaryngology—Head and Neck Surgery. 16th ed. BC Decker Inc.; Hamilton, ON, Canada: 2003. pp. 249–260.
Grants and funding
LinkOut - more resources
Full Text Sources

