The Effect of Mesenchymal Stem Cells, Adipose Tissue Derived Stem Cells, and Cellular Stromal Vascular Fraction on the Repair of Acute Anal Sphincter Injury in Rats
- PMID: 35877369
- PMCID: PMC9311655
- DOI: 10.3390/bioengineering9070318
The Effect of Mesenchymal Stem Cells, Adipose Tissue Derived Stem Cells, and Cellular Stromal Vascular Fraction on the Repair of Acute Anal Sphincter Injury in Rats
Abstract
Background: Anal sphincter incontinence (ASI) can cause a serious decline in the quality of life and can cause a socioeconomic burden. Studies have shown that bone marrow mesenchymal stem cells (MSC) have significant therapeutic effects on ASI, but the cost and risk of MSC harvest limit their further application. In contrast, adipose tissue derived stem cells (ADSC) and cellular stromal vascular fraction (CSVF) as stem cell sources have multipotency and the advantage of easy harvest.
Objective: Here we aim to investigate the effects of ADSC and CSVF on treating ASI and compare them to that of bone marrow MSC.
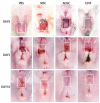
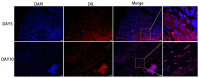
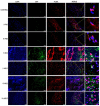
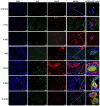
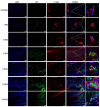
Methods: Bone marrow MSC, ADSC, and CSVF were obtained and labeled with green fluorescent protein (GFP), and CSVF was labeled with DIL. Sprague Dawley (SD) rats were divided into 5 groups. Four groups were injected with 0.2 mL phosphate buffer saline (PBS), 1 × 107/0.2 mL of MSC, ADSC, or CSVF, respectively, after model establishment. The control group received no treatment. The repair was assessed by anal functional tests and immunostaining on day 5 and day 10 after injection.
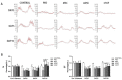
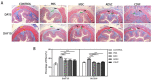
Results: MSC, ADSC, and CSVF significantly promoted tissue repair and the recovery of muscle contraction and electromyographic activity in ASI. The generation of myosatellite cells by injected MSC, ADSC, and CSVF was found in the wounded area. On day 5, CSVF showed highest therapeutic effect, while on day 10, MSC and ADSC showed higher therapeutic effects than CSVF. When comparing the effects of MSC and ADSC, ADSC was slightly better than MSC in the indexes of anal pressure, etc. Conclusion: ADSC and CVSF are alternative stem cell sources for ASI repair.
Keywords: ADSC; ASI; CSVF; MSC; anal sphincter incontinence.
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
The Effect of Tissue Stromal Vascular Fraction as Compared to Cellular Stromal Vascular Fraction to Treat Anal Sphincter Incontinence.Bioengineering (Basel). 2022 Dec 26;10(1):32. doi: 10.3390/bioengineering10010032. Bioengineering (Basel). 2022. PMID: 36671604 Free PMC article.
-
Local injection of bone marrow progenitor cells for the treatment of anal sphincter injury: in-vitro expanded versus minimally-manipulated cells.Stem Cell Res Ther. 2016 Jun 21;7(1):85. doi: 10.1186/s13287-016-0344-x. Stem Cell Res Ther. 2016. PMID: 27328811 Free PMC article.
-
Local transplantation of syngeneic adipose stromal vascular fraction ameliorates damaged anal sphincter function in a rat model of vaginal distension.Surgery. 2022 Oct;172(4):1093-1101. doi: 10.1016/j.surg.2022.06.015. Epub 2022 Aug 14. Surgery. 2022. PMID: 35973873
-
Defining adipose tissue-derived stem cells in tissue and in culture.Histol Histopathol. 2010 Jun;25(6):807-15. doi: 10.14670/HH-25.807. Histol Histopathol. 2010. PMID: 20376787 Review.
-
Is CD34 truly a negative marker for mesenchymal stromal cells?Cytotherapy. 2012 Nov;14(10):1159-63. doi: 10.3109/14653249.2012.729817. Cytotherapy. 2012. PMID: 23066784 Free PMC article. Review.
Cited by
-
Human umbilical cord mesenchymal stem cells on treating osteoarthritis in a rabbit model: Injection strategies.Heliyon. 2024 Sep 28;10(19):e38384. doi: 10.1016/j.heliyon.2024.e38384. eCollection 2024 Oct 15. Heliyon. 2024. PMID: 39430502 Free PMC article.
-
Analyzing the Clinical Potential of Stromal Vascular Fraction: A Comprehensive Literature Review.Medicina (Kaunas). 2024 Jan 27;60(2):221. doi: 10.3390/medicina60020221. Medicina (Kaunas). 2024. PMID: 38399509 Free PMC article.
-
Stem cell therapy combined with controlled release of growth factors for the treatment of sphincter dysfunction.Cell Biosci. 2023 Mar 16;13(1):56. doi: 10.1186/s13578-023-01009-3. Cell Biosci. 2023. PMID: 36927578 Free PMC article. Review.
-
Therapeutic potential of stem cell-derived exosomes for bone tissue regeneration around prostheses.J Orthop Translat. 2025 Apr 11;52:85-96. doi: 10.1016/j.jot.2025.03.007. eCollection 2025 May. J Orthop Translat. 2025. PMID: 40291635 Free PMC article. Review.
-
Nano wear particles and the periprosthetic microenvironment in aseptic loosening induced osteolysis following joint arthroplasty.Front Cell Infect Microbiol. 2023 Oct 3;13:1275086. doi: 10.3389/fcimb.2023.1275086. eCollection 2023. Front Cell Infect Microbiol. 2023. PMID: 37854857 Free PMC article. Review.
References
Grants and funding
- SYPTKTA2021041/Guangdong Natural Science Foundation
- SYPTKTB2019058/Guangzhou science and Technology Program
- 2019TS52/Special Clinical Technology Program of Guangzhou
- 2020A1515011031/Natural Science Foundation of Guangdong Province
- 2020B1111170004/Guangdong Provincial Clinical Research Center for Digestive Diseases
LinkOut - more resources
Full Text Sources

