The potential of a nomogram combined PI-RADS v2.1 and contrast-enhanced ultrasound (CEUS) to reduce unnecessary biopsies in prostate cancer diagnostics
- PMID: 35877385
- PMCID: PMC9815734
- DOI: 10.1259/bjr.20220209
The potential of a nomogram combined PI-RADS v2.1 and contrast-enhanced ultrasound (CEUS) to reduce unnecessary biopsies in prostate cancer diagnostics
Abstract
Objectives: To develop a nomogram prediction model based on Prostate Imaging Reporting and Data System v.2.1 (PI-RADS v2.1) and contrast-enhanced ultrasound (CEUS) for predicting prostate cancer (PCa) and clinically significant prostate cancer (csPCa) in males with prostate-specific antigen (PSA) 4-10 ng ml-1 to avoid unnecessary biopsy.
Methods: A total of 490 patients who underwent prostate biopsy for PSA 4-10 ng ml-1 were enrolled and randomly divided into a pilot cohort (70%) and a validation cohort (30%). Univariate and multivariate logistic regression models were constructed to select potential predictors of PCa and csPCa, and a nomogram was created. The area under receiver operating characteristic (ROC) curve (AUC) was calculated, and compared using DeLong's test. The diagnostic performance and unnecessary biopsy rate of the nomogram prediction model were also assessed. Hosmer-Lemeshow goodness-of-fit test was employed to test for model fitness.
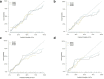
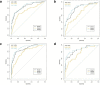
Results: The multivariate analysis revealed that features independently associated with PCa and csPCa were age, PI-RADS score and CEUS manifestations. Incorporating these factors, the nomogram achieved good discrimination performance of AUC 0.843 for PCa, 0.876 for csPCa in the pilot cohort, and 0.818 for PCa, 0.857 for csPCa in the validation cohort, respectively, and had well-fitted calibration curves. And the diagnostic performance of the nomogram was comparable to the model including all the parameters (p > 0.05). Besides, the nomogram prediction model yielded meaningful reduction in unnecessary biopsy rate (from 74.8 to 21.1% in PCa, and from 83.7 to 5.4% in csPCa).
Conclusions: The nomogram prediction model based on age, PI-RADS v2.1 and CEUS achieved an optimal prediction of PCa and csPCa. Using this model, the PCa risk for an individual patient can be estimated, which can lead to a rational biopsy choice.
Advances in knowledge: This study gives an account of improving pre-biopsy risk stratification in males with "gray zone" PSA level through PI-RADS v2.1 and CEUS.
Conflict of interest statement
Figures

Similar articles
-
Development of a nomogram combining multiparametric magnetic resonance imaging and PSA-related parameters to enhance the detection of clinically significant cancer across different region.Prostate. 2022 Apr;82(5):556-565. doi: 10.1002/pros.24302. Epub 2022 Jan 31. Prostate. 2022. PMID: 35098557
-
How to make clinical decisions to avoid unnecessary prostate screening in biopsy-naïve men with PI-RADs v2 score ≤ 3?Int J Clin Oncol. 2020 Jan;25(1):175-186. doi: 10.1007/s10147-019-01524-9. Epub 2019 Aug 31. Int J Clin Oncol. 2020. PMID: 31473884
-
Development and external validation of a novel nomogram to predict prostate cancer in biopsy-naïve patients with PSA <10 ng/ml and PI-RADS v2.1 = 3 lesions.Cancer Med. 2023 Feb;12(3):2560-2571. doi: 10.1002/cam4.5100. Epub 2022 Aug 3. Cancer Med. 2023. PMID: 35920264 Free PMC article.
-
Magnetic Resonance Imaging, Clinical, and Biopsy Findings in Suspected Prostate Cancer: A Systematic Review and Meta-Analysis.JAMA Netw Open. 2024 Mar 4;7(3):e244258. doi: 10.1001/jamanetworkopen.2024.4258. JAMA Netw Open. 2024. PMID: 38551559 Free PMC article.
-
Magnetic Resonance Imaging-Based Predictive Models for Clinically Significant Prostate Cancer: A Systematic Review.Cancers (Basel). 2022 Sep 29;14(19):4747. doi: 10.3390/cancers14194747. Cancers (Basel). 2022. PMID: 36230670 Free PMC article. Review.
Cited by
-
Advances in radiology and pathology of prostate cancer: a review for the pathologist.Pathologica. 2024 Feb;116(1):1-12. doi: 10.32074/1591-951X-925. Epub 2024 Feb 8. Pathologica. 2024. PMID: 38349336 Free PMC article. Review.
-
Comparative Assessment of Different Ultrasound Technologies in the Detection of Prostate Cancer: A Systematic Review and Meta-Analysis.Cancers (Basel). 2023 Aug 15;15(16):4105. doi: 10.3390/cancers15164105. Cancers (Basel). 2023. PMID: 37627133 Free PMC article. Review.
-
Value of machine learning-based transrectal multimodal ultrasound combined with PSA-related indicators in the diagnosis of clinically significant prostate cancer.Front Endocrinol (Lausanne). 2023 Mar 8;14:1137322. doi: 10.3389/fendo.2023.1137322. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 36967794 Free PMC article.
-
Development and validation of a nomogram for predicting prostate cancer based on combining contrast-enhanced transrectal ultrasound and biparametric MRI imaging.Front Oncol. 2023 Nov 17;13:1275773. doi: 10.3389/fonc.2023.1275773. eCollection 2023. Front Oncol. 2023. PMID: 38044995 Free PMC article.
-
PI-RADS v2.1 evaluation of prostate "nodule in nodule" variants: clinical, imaging, and pathological features.Insights Imaging. 2024 Mar 18;15(1):79. doi: 10.1186/s13244-024-01651-6. Insights Imaging. 2024. PMID: 38499703 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

