Nucleocapsid Antigenemia Is a Marker of Acute SARS-CoV-2 Infection
- PMID: 35877413
- PMCID: PMC9384592
- DOI: 10.1093/infdis/jiac225
Nucleocapsid Antigenemia Is a Marker of Acute SARS-CoV-2 Infection
Abstract
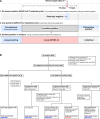
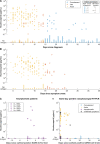
Detecting severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection is essential for diagnosis, treatment, and infection control. Polymerase chain reaction (PCR) fails to distinguish acute from resolved infections, as RNA is frequently detected after infectiousness. We hypothesized that nucleocapsid in blood marks acute infection with the potential to enhance isolation and treatment strategies. In a retrospective serosurvey of inpatient and outpatient encounters, we categorized samples along an infection timeline using timing of SARS-CoV-2 testing and symptomatology. Among 1860 specimens from 1607 patients, the highest levels and frequency of antigenemia were observed in samples from acute SARS-CoV-2 infection. Antigenemia was higher in seronegative individuals and in those with severe disease. In our analysis, antigenemia exhibited 85.8% sensitivity and 98.6% specificity as a biomarker for acute coronavirus disease 2019 (COVID-19). Thus, antigenemia sensitively and specifically marks acute SARS-CoV-2 infection. Further study is warranted to determine whether antigenemia may aid individualized assessment of active COVID-19.
Keywords: COVID-19; SARS-CoV-2; antigenemia; nucleocapsid.
© The Author(s) 2022. Published by Oxford University Press on behalf of Infectious Diseases Society of America. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Conflict of interest statement
Potential conflicts of interest. C. A. R.’s institution has received funds to conduct clinical research unrelated to this work from BioFire Inc, GSK, MedImmune, Micron, Janssen, Merck, Moderna, Novavax, PaxVax, Pfizer, Regeneron, and Sanofi-Pasteur; she is coinventor of patented RSV vaccine technology unrelated to this work, which has been licensed to Meissa Vaccines, Inc. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Figures



References
-
- Centers for Disease Control and Prevention . COVID-19 quarantine and isolation. https://www.cdc.gov/coronavirus/2019-ncov/your-health/quarantine-isolati.... Accessed 29 November 2021.
-
- Centers for Disease Control and Prevention . CDC updates and shortens recommended isolation and quarantine period for general population. CDC Newsroom Releases; 2021. https://www.cdc.gov/media/releases/2021/s1227-isolation-quarantine-guida.... Accessed 29 November 2021.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

