Identification of Putative Plant-Based ALR-2 Inhibitors to Treat Diabetic Peripheral Neuropathy
- PMID: 35877418
- PMCID: PMC9319673
- DOI: 10.3390/cimb44070194
Identification of Putative Plant-Based ALR-2 Inhibitors to Treat Diabetic Peripheral Neuropathy
Abstract
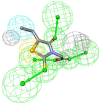
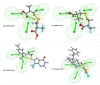
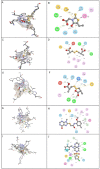
Diabetic peripheral neuropathy (DPN) is a common diabetes complication (DM). Aldose reductase -2 (ALR-2) is an oxidoreductase enzyme that is most extensively studied therapeutic target for diabetes-related complications that can be inhibited by epalrestat, which has severe adverse effects; hence the discovery of potent natural inhibitors is desired. In response, a pharmacophore model based on the properties of eplarestat was generated. The specified pharmacophore model searched the NuBBEDB database of natural compounds for prospective lead candidates. To assess the drug-likeness and ADMET profile of the compounds, a series of in silico filtering procedures were applied. The compounds were then put through molecular docking and interaction analysis. In comparison to the reference drug, four compounds showed increased binding affinity and demonstrated critical residue interactions with greater stability and specificity. As a result, we have identified four potent inhibitors: ZINC000002895847, ZINC000002566593, ZINC000012447255, and ZINC000065074786, that could be used as pharmacological niches to develop novel ALR-2 inhibitors.
Keywords: ADMET; NuBBEDB; molecular docking; pharmacophore; structure-based drug design.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Yang H.-Y., Li Q.-R., Wang Z., Zhou W., Fan S.-R., Ma R., Xue L., Yang L., Li Y.-S., Tan H.-L., et al. Epalrestat protects against diabetic peripheral neuropathy by alleviating oxidative stress and inhibiting polyol pathway. Neural Regen. Res. 2016;11:345–351. doi: 10.4103/1673-5374.177745. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

