Design, Synthesis, and Biological Evaluations of Novel Azothiazoles Based on Thioamide
- PMID: 35877428
- PMCID: PMC9323687
- DOI: 10.3390/cimb44070204
Design, Synthesis, and Biological Evaluations of Novel Azothiazoles Based on Thioamide
Abstract
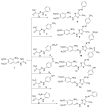
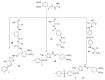
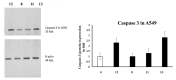
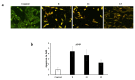
Herein we studied the preparation of different thiazoles via the reaction of 2-(3,4-dimethoxybenzylidene)hydrazine-1-carbothioamide (1) with hydrazonoyl halides under base-catalyzed conditions. The reactions proceed through nucleophilic substitution attack at the halogen atom of the hydrazonoyl halides by the thiol nucleophile to form an S-alkylated intermediate. The latter intermediate undergoes cyclization by the loss of water to afford the final products. The structures of the azo compounds were confirmed by FTIR, MS, NMR, and elemental analyses. Indeed, the newly synthesized azo compounds were estimated for their potential anticancer activities by an MTT assay against different human cancer cells, such as lung adenocarcinoma (A549) and colorectal adenocarcinoma (DLD-1). The caspase-3 levels were also estimated using Western blotting and the dual staining technique to evaluate the potency of the titled compounds to promote apoptosis.
Keywords: anticancer activities; carbothioamide; hydrazonoyl; thiazole.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

Similar articles
-
Efficient Methods for the Synthesis of Novel Arylazothiazoles Based on Acetylferrocene or Adamantane.Curr Org Synth. 2020;17(4):282-287. doi: 10.2174/1570179417666200226091711. Curr Org Synth. 2020. PMID: 32101128
-
Biological Activity, Apoptotic Induction and Cell Cycle Arrest of New Hydrazonoyl Halides Derivatives.Anticancer Agents Med Chem. 2019;19(9):1141-1149. doi: 10.2174/1871520619666190306123658. Anticancer Agents Med Chem. 2019. PMID: 30843494
-
Utility of 5-(furan-2-yl)-3-(p-tolyl)-4,5-dihydro-1H-pyrazole-1-carbothioamide in the synthesis of heterocyclic compounds with antimicrobial activity.BMC Chem. 2019 Apr 1;13(1):48. doi: 10.1186/s13065-019-0566-y. eCollection 2019 Dec. BMC Chem. 2019. PMID: 31384796 Free PMC article.
-
A facile synthesis and anticancer activity of some novel thiazoles carrying 1,3,4-thiadiazole moiety.Chem Cent J. 2017 Mar 21;11(1):25. doi: 10.1186/s13065-017-0255-7. Chem Cent J. 2017. PMID: 29086817 Free PMC article.
-
A review on bis-hydrazonoyl halides: Recent advances in their synthesis and their diverse synthetic applications leading to bis-heterocycles of biological interest.J Adv Res. 2016 Nov;7(6):873-907. doi: 10.1016/j.jare.2016.09.001. Epub 2016 Sep 13. J Adv Res. 2016. PMID: 27672450 Free PMC article. Review.
Cited by
-
An Efficient Synthesis of Novel Aminothiazolylacetamido-Substituted 3,5-Bis(arylidene)-4-piperidone Derivatives and Their Cytotoxicity Studies.ACS Omega. 2024 Jun 26;9(27):29244-29251. doi: 10.1021/acsomega.4c00039. eCollection 2024 Jul 9. ACS Omega. 2024. PMID: 39005779 Free PMC article.
-
Reactions and Biological Activities of Hydrazonoyl Halides.Curr Org Synth. 2024;21(8):1021-1052. doi: 10.2174/0115701794268313231127110713. Curr Org Synth. 2024. PMID: 39044696
References
-
- Chankhanittha T., Somaudon V., Watcharakitti J., Piyavarakorn V., Nanan S. Performance of solvothermally grown Bi2MoO6 photocatalyst toward degradation of organic azo dyes and fluoroquinolone antibiotics. Mater. Lett. 2020;258:126764. doi: 10.1016/j.matlet.2019.126764. - DOI
-
- Gaffer H.E. Antimicrobial sulphonamide azo dyes. Color. Technol. 2019;135:484–500. doi: 10.1111/cote.12437. - DOI
-
- Ghasemi Z., Azizi S., Salehi R., Kafil H.S. Synthesis of azo dyes possessing N-heterocycles and evaluation of their anticancer and antibacterial properties. Monatsh. Chem. 2018;149:149–157. doi: 10.1007/s00706-017-2073-y. - DOI
-
- Afifi T.H., Okasha R.M., Ahmed H.E., Ilaš J., Saleh T., Abd-El-Aziz A.S. Structure-activity relationships and molecular docking studies of chromene and chromene based azo chromophores: A novel series of potent antimicrobial and anticancer agents. EXCLI J. 2017;16:868–902. doi: 10.17179/excli2017-356. - DOI - PMC - PubMed
-
- Gaffer H.E., Elgohary M.R., Etman H.A., Shaaban S. Antibacterial evaluation of cotton fabrics by using novel sulfonamide reactive dyes. Pigm. Resin Technol. 2017;46:210–217. doi: 10.1108/PRT-08-2015-0080. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

