Mucoadhesive and Antimicrobial Allantoin/β Cyclodextrins-Loaded Carbopol Gels as Scaffolds for Regenerative Medicine
- PMID: 35877501
- PMCID: PMC9320337
- DOI: 10.3390/gels8070416
Mucoadhesive and Antimicrobial Allantoin/β Cyclodextrins-Loaded Carbopol Gels as Scaffolds for Regenerative Medicine
Abstract
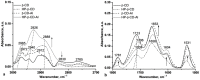
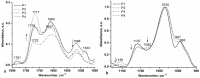
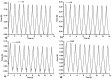
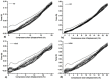
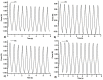
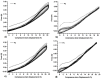
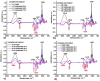
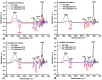
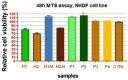
Allantoin and its β-cyclodextrin and hydroxypropyl-β-cyclodextrin inclusion complexes 1:1 have been used to prepare carbopol-based mucoadhesive gels. The gelation process occurred by adjustment with glycerol 10% in the presence of triethanolamine. The structural features induced by the presence of allantoin and the corresponding β-cyclodextrins inclusion complexes have been first investigated by infrared spectroscopy highlighting strong interactions within the gels network and ideal crosslinks for the self-healing behavior. The hydrophilicity of the gels was investigated by the determination of the surface tension parameters and the free energy of hydration. The interfacial free energy values indicated prolonged biocompatibility with blood. The gels-water molecule interactions in terms of sorption, permeability, and diffusion coefficients were evaluated by dynamic vapor sorption analysis. The self-assembly process through intermolecular H-bonding, the high hydrophilicity, the mechanical performance, the hydrolytic stability in simulated biological media, the biocompatibility with normal human dermal fibroblast (NHDF) cells, the mucoadhesivity and antimicrobial activity on selected microorganism species (S. Aureus and C. albicans) of the allantoin-based gels recommend them as promising scaffold alternatives in regenerative medicine.
Keywords: antibacterial; interfacial energy; mucoadhesion; tissue engineering scaffolds; β-cyclodextrin/allantoin inclusion complexes.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Hayati F., Ghamsari S.M., Tavassoli A., Azizzadeh M. Carbomer 940 Hydrogel Enhances Capillary Blood Flow and Tissue Viability in a Skin Burn Wound. IJVS. 2016;11:29–36.
-
- Huang L., Wang J., Huang S., Siaw-Debrah F., Nyanzu M., Zhuge Q. Polyacrylic Acid-Coated Nanoparticles Loaded with Recombinant Tissue Plasminogen Activator for the Treatment of Mice with Ischemic Stroke. Biochem. Biophys. Res. Commun. 2019;516:565–570. doi: 10.1016/j.bbrc.2019.06.079. - DOI - PubMed
-
- Larsson M., Bergstrand A., Mesiah L., Van Vooren C., Larsson A. Nanocomposites of Polyacrylic Acid Nanogels and Biodegradable Polyhydroxybutyrate for Bone Regeneration and Drug Delivery. J. Nanomater. 2014;2014:371307. doi: 10.1155/2014/371307. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

