Formulation and Evaluation of Moxifloxacin Loaded Bilosomes In-Situ Gel: Optimization to Antibacterial Evaluation
- PMID: 35877503
- PMCID: PMC9323078
- DOI: 10.3390/gels8070418
Formulation and Evaluation of Moxifloxacin Loaded Bilosomes In-Situ Gel: Optimization to Antibacterial Evaluation
Abstract
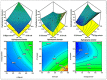
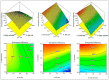
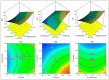
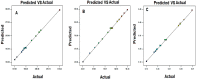
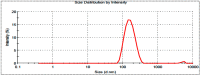
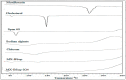
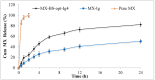
In this study, moxifloxacin (MX)-loaded bilosome (BS) in situ gel was prepared to improve ocular residence time. MX-BSs were prepared using the thin-film hydration method. They were optimized using a Box−Behnken design (BBD) with bile salt (A, sodium deoxycholate), an edge activator (B, Cremophor EL), and a surfactant (C, Span 60) as process variables. Their effects were assessed based on hydrodynamic diameter (Y1), entrapment efficacy (Y2), and polydispersity index (Y3). The optimized formulation (MX-BSop) depicted a low hydrodynamic diameter (192 ± 4 nm) and high entrapment efficiency (76 ± 1%). Further, MX-BSop was successfully transformed into an in situ gel using chitosan and sodium alginate as carriers. The optimized MX-BSop in situ gel (MX-BSop-Ig4) was further evaluated for gelling capacity, clarity, pH, viscosity, in vitro release, bio-adhesiveness, ex vivo permeation, toxicity, and antimicrobial properties. MX-BSop-Ig4 exhibited an optimum viscosity of 65.4 ± 5.3 cps in sol and 287.5 ± 10.5 cps in gel states. The sustained release profile (82 ± 4% in 24 h) was achieved with a Korsmeyer−Peppas kinetic release model (R2 = 0.9466). Significant bio-adhesion (967.9 dyne/cm2) was achieved in tear film. It also exhibited 1.2-fold and 2.8-fold higher permeation than MX-Ig and a pure MX solution, respectively. It did not show any toxicity to the tested tissue, confirmed by corneal hydration (77.3%), cornea histopathology (no internal changes), and a HET-CAM test (zero score). MX-BSop-Ig4 exhibited a significantly (p < 0.05) higher antimicrobial effect than pure MX against Staphylococcus aureus and Escherichia coli. The findings suggest that bilosome in situ gel is a good alternative to increase corneal residence time, as well as to improve therapeutic activity.
Keywords: antimicrobial activity; bilosomes; moxifloxacin; ocular delivery; toxicity study.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Development of Ciprofloxacin-Loaded Bilosomes In-Situ Gel for Ocular Delivery: Optimization, In-Vitro Characterization, Ex-Vivo Permeation, and Antimicrobial Study.Gels. 2022 Oct 25;8(11):687. doi: 10.3390/gels8110687. Gels. 2022. PMID: 36354595 Free PMC article.
-
Development of Gentamicin Bilosomes Laden In Situ Gel for Topical Ocular Delivery: Optimization, In Vitro Characterization, Toxicity, and Anti-microbial Evaluation.Adv Pharm Bull. 2024 Oct;14(3):646-664. doi: 10.34172/apb.2024.057. Epub 2024 Jul 31. Adv Pharm Bull. 2024. PMID: 39494264 Free PMC article.
-
Clarithromycin-Loaded Ocular Chitosan Nanoparticle: Formulation, Optimization, Characterization, Ocular Irritation, and Antimicrobial Activity.Int J Nanomedicine. 2020 Oct 13;15:7861-7875. doi: 10.2147/IJN.S269004. eCollection 2020. Int J Nanomedicine. 2020. PMID: 33116505 Free PMC article.
-
Preparation of NLCs-Based Topical Erythromycin Gel: In Vitro Characterization and Antibacterial Assessment.Gels. 2022 Feb 13;8(2):116. doi: 10.3390/gels8020116. Gels. 2022. PMID: 35200497 Free PMC article.
-
Formulation of Chitosan Polymeric Vesicles of Ciprofloxacin for Ocular Delivery: Box-Behnken Optimization, In Vitro Characterization, HET-CAM Irritation, and Antimicrobial Assessment.AAPS PharmSciTech. 2020 Jun 5;21(5):167. doi: 10.1208/s12249-020-01699-9. AAPS PharmSciTech. 2020. PMID: 32504176
Cited by
-
Moxifloxacin HCl-Incorporated Aqueous-Induced Nitrocellulose-Based In Situ Gel for Periodontal Pocket Delivery.Gels. 2023 Jul 13;9(7):572. doi: 10.3390/gels9070572. Gels. 2023. PMID: 37504451 Free PMC article.
-
Development and optimization of dapagliflozin oral nano-bilosomes using response surface method: in vitro evaluation, in vivo evaluation.Nanotheranostics. 2025 Jan 1;9(1):1-19. doi: 10.7150/ntno.99271. eCollection 2025. Nanotheranostics. 2025. PMID: 39744098 Free PMC article.
-
Bilosomes as Nanocarriers for the Drug and Vaccine Delivery against Gastrointestinal Infections: Opportunities and Challenges.J Funct Biomater. 2023 Sep 1;14(9):453. doi: 10.3390/jfb14090453. J Funct Biomater. 2023. PMID: 37754867 Free PMC article. Review.
-
Nanovesicular Drug Delivery Systems for Rare Ocular Diseases: Advances, Challenges, and Future Directions.AAPS PharmSciTech. 2025 Jul 23;26(7):197. doi: 10.1208/s12249-025-03159-8. AAPS PharmSciTech. 2025. PMID: 40702295 Review.
-
Formulation, optimization, in-vivo biodistribution studies and histopathological safety assessment of duloxetine HCl-loaded ultra-elastic nanovesicles for antidepressant effect after intranasal and transdermal delivery.Int J Pharm X. 2023 Jun 24;6:100194. doi: 10.1016/j.ijpx.2023.100194. eCollection 2023 Dec 15. Int J Pharm X. 2023. PMID: 37434966 Free PMC article.
References
-
- Souto E.B., Dias-Ferreira J., López-Machado A., Ettcheto M., Cano A., Espuny A.C., Espina M., Garcia M.L., Sánchez-López E. Advanced Formulation Approaches for Ocular Drug Delivery: State-Of-The-Art and Recent Patents. Pharmaceutics. 2019;11:460. doi: 10.3390/pharmaceutics11090460. - DOI - PMC - PubMed
-
- Polat H.K., Pehlivan S.B., Özkul C., Calamak S., Ozturk N., Aytekin E., Fırat A., Ulubayram K., Kocabeyoğlu S., Irkeç M., et al. Development of besifloxacin HCl loaded nanofibrous ocular inserts for the treatment of bacterial keratitis: In vitro, ex vivo and in vivo evaluation. Int. J. Pharm. 2020;585:119552. doi: 10.1016/j.ijpharm.2020.119552. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

