Poly(N-isopropylacrylamide)-Based Hydrogels for Biomedical Applications: A Review of the State-of-the-Art
- PMID: 35877539
- PMCID: PMC9323937
- DOI: 10.3390/gels8070454
Poly(N-isopropylacrylamide)-Based Hydrogels for Biomedical Applications: A Review of the State-of-the-Art
Abstract
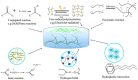
A prominent research topic in contemporary advanced functional materials science is the production of smart materials based on polymers that may independently adjust their physical and/or chemical characteristics when subjected to external stimuli. Smart hydrogels based on poly(N-isopropylacrylamide) (PNIPAM) demonstrate distinct thermoresponsive features close to a lower critical solution temperature (LCST) that enhance their capability in various biomedical applications such as drug delivery, tissue engineering, and wound dressings. Nevertheless, they have intrinsic shortcomings such as poor mechanical properties, limited loading capacity of actives, and poor biodegradability. Formulation of PNIPAM with diverse functional constituents to develop hydrogel composites is an efficient scheme to overcome these defects, which can significantly help for practicable application. This review reports on the latest developments in functional PNIPAM-based smart hydrogels for various biomedical applications. The first section describes the properties of PNIPAM-based hydrogels, followed by potential applications in diverse fields. Ultimately, this review summarizes the challenges and opportunities in this emerging area of research and development concerning this fascinating polymer-based system deep-rooted in chemistry and material science.
Keywords: PNIPAM; drug delivery; hydrogels; smart polymer; tissue engineering; wound healing.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Smart Poly(N-isopropylacrylamide)-Based Hydrogels: A Tour D'horizon of Biomedical Applications.Gels. 2025 Mar 15;11(3):207. doi: 10.3390/gels11030207. Gels. 2025. PMID: 40136912 Free PMC article. Review.
-
Poly(N-isopropylacrylamide)-Based Thermoresponsive Composite Hydrogels for Biomedical Applications.Polymers (Basel). 2020 Mar 5;12(3):580. doi: 10.3390/polym12030580. Polymers (Basel). 2020. PMID: 32150904 Free PMC article. Review.
-
Mechanical properties of PNIPAM based hydrogels: A review.Mater Sci Eng C Mater Biol Appl. 2017 Jan 1;70(Pt 1):842-855. doi: 10.1016/j.msec.2016.09.081. Epub 2016 Sep 30. Mater Sci Eng C Mater Biol Appl. 2017. PMID: 27770962 Review.
-
Refined control of thermoresponsive swelling/deswelling and drug release properties of poly(N-isopropylacrylamide) hydrogels using hydrophilic polymer crosslinkers.J Biomater Sci Polym Ed. 2016 Dec;27(17):1698-1711. doi: 10.1080/09205063.2016.1230933. Epub 2016 Sep 11. J Biomater Sci Polym Ed. 2016. PMID: 27573586
-
Phase transition and potential biomedical applications of thermoresponsive compositions based on polysaccharides, proteins and DNA: A review.Int J Biol Macromol. 2023 Sep 30;249:126054. doi: 10.1016/j.ijbiomac.2023.126054. Epub 2023 Jul 31. Int J Biol Macromol. 2023. PMID: 37532189 Review.
Cited by
-
Recent advances of injectable in situ-forming hydrogels for preventing postoperative tumor recurrence.Drug Deliv. 2024 Dec;31(1):2400476. doi: 10.1080/10717544.2024.2400476. Epub 2024 Sep 10. Drug Deliv. 2024. PMID: 39252545 Free PMC article. Review.
-
A New Design of Poly(N-Isopropylacrylamide) Hydrogels Using Biodegradable Poly(Beta-Aminoester) Crosslinkers as Fertilizer Reservoirs for Agricultural Applications.Gels. 2023 Feb 3;9(2):127. doi: 10.3390/gels9020127. Gels. 2023. PMID: 36826297 Free PMC article.
-
Efficient Cross-Linking through C-H Bond Insertion of Unfunctionalized Commodity Materials Using Diazirine-Containing Polymers.ACS Macro Lett. 2024 Nov 19;13(11):1598-1604. doi: 10.1021/acsmacrolett.4c00675. Epub 2024 Nov 8. ACS Macro Lett. 2024. PMID: 39513545 Free PMC article.
-
Current Progress and Emerging Role of Essential Oils in Drug Delivery Therapeutics.Curr Drug Deliv. 2025;22(3):332-357. doi: 10.2174/0115672018287719240214075810. Curr Drug Deliv. 2025. PMID: 38409707 Review.
-
Biomedical Application of Nanogels: From Cancer to Wound Healing.Molecules. 2025 May 13;30(10):2144. doi: 10.3390/molecules30102144. Molecules. 2025. PMID: 40430316 Free PMC article. Review.
References
-
- Shrivastav P., Pramanik S., Vaidya G., Abdelgawad M.A., Ghoneim M.M., Singh A., Abualsoud B.M., Amaral L.S., Abourehab M.A. Bacterial cellulose as a potential biopolymer in biomedical applications: A state-of-the-art review. J. Mater. Chem. B. 2022;10:3199–3241. doi: 10.1039/D1TB02709C. - DOI - PubMed
-
- Ferreira N.N., Ferreira L.M.B., Cardoso V.M.O., Boni F.I., Souza A.L.R., Gremião M.P.D. Recent advances in smart hydrogels for biomedical applications: From self-assembly to functional approaches. Eur. Polym. J. 2018;99:117–133. doi: 10.1016/j.eurpolymj.2017.12.004. - DOI
-
- Jonker A.M., Löwik D.W.P.M., van Hest J.C.M. Peptide- and protein-based hydrogels. Chem. Mater. 2012;24:759–773. doi: 10.1021/cm202640w. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

