Low Prevalence of Cardiomyopathy in Patients with Mitochondrial Disease and Neurological Manifestations
- PMID: 35877583
- PMCID: PMC9320353
- DOI: 10.3390/jcdd9070221
Low Prevalence of Cardiomyopathy in Patients with Mitochondrial Disease and Neurological Manifestations
Abstract
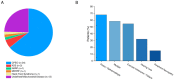
Patients with mitochondrial diseases can develop cardiomyopathy but with variable expressivity and penetrance. Our prospective study enrolled and evaluated a cohort of 53 patients diagnosed with chronic progressive ophthalmoplegia (CPEO, n = 34), Kearns-Sayre syndrome (KSS, n = 3), neuropathy ataxia and retinitis pigmentosa (NARP, n = 1), myoclonic epilepsy with ragged red fibers (MERRF, n = 1), Harel-Yoon Syndrome (HYS, n = 1) and 13 patients with undefined mitochondrial diseases, presenting primarily with neurological symptoms. Over a 4-year period, six patients in our study cohort were diagnosed with heart disease (11.3%), with only three patients having defined cardiomyopathy (5.7%). Cardiomyopathy was present in a 21-year-old patient with HYS and two CPEO patients having mild cardiomyopathy at an older age. Two CPEO patients had congenital heart disease, and a third CPEO had LV hypertrophy secondary to hypertension. In three patients, traditional risk factors for heart disease, including dyslipidemia, hypertension, and respiratory disease, were present. The majority of our adult cohort of patients have normal cardiac investigations with a median left ventricular (LV) ejection fraction of 59.0%, indexed LV mass of 67.0 g/m2, and normal diastolic and valvular function at baseline. A 12-lead electrocardiogram showed normal cardiac conduction across the study cohort. Importantly, follow-up assessments showed consistent cardiac structure and function. Our study shows a low prevalence of cardiomyopathy and highlights the breadth of phenotypic variability in patients with mitochondrial disorders. The presence of cardiovascular risk factors and aging are important comorbidities in our cohort.
Keywords: cardiac imaging; cardiomyopathy; heterogeneity; mitochondrial disease.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Grants and funding
LinkOut - more resources
Full Text Sources

