Cytidinediphosphate diacylglycerol synthase-Mediated phosphatidic acid metabolism is crucial for early embryonic development of Arabidopsis
- PMID: 35877676
- PMCID: PMC9352201
- DOI: 10.1371/journal.pgen.1010320
Cytidinediphosphate diacylglycerol synthase-Mediated phosphatidic acid metabolism is crucial for early embryonic development of Arabidopsis
Abstract
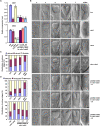
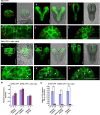
Embryonic development is a key developmental event in plant sexual reproduction; however, regulatory networks of plant early embryonic development, particularly the effects and functional mechanisms of phospholipid molecules are still unknown due to the limitation of sample collection and analysis. We innovatively applied the microspore-derived in vitro embryogenesis of Brassica napus and revealed the dynamics of phospholipid molecules, especially phosphatidic acid (PA, an important second messenger that plays an important role in plant growth, development, and stress responses), at different embryonic developmental stages by using a lipidomics approach. Further analysis of Arabidopsis mutants deficiency of CDS1 and CDS2 (cytidinediphosphate diacylglycerol synthase, key protein in PA metabolism) revealed the delayed embryonic development from the proembryo stage, indicating the crucial effect of CDS and PA metabolism in early embryonic development. Decreased auxin level and disturbed polar localization of auxin efflux carrier PIN1 implicate that CDS-mediated PA metabolism may regulate early embryogenesis through modulating auxin transport and distribution. These results demonstrate the dynamics and importance of phospholipid molecules during embryo development, and provide informative clues to elucidate the regulatory network of embryogenesis.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



Similar articles
-
Extraplastidial cytidinediphosphate diacylglycerol synthase activity is required for vegetative development in Arabidopsis thaliana.Plant J. 2013 Sep;75(5):867-79. doi: 10.1111/tpj.12248. Epub 2013 Jun 21. Plant J. 2013. PMID: 23711240
-
Phosphatidic acid is a major phospholipid class in reproductive organs of Arabidopsis thaliana.Plant Signal Behav. 2015;10(8):e1049790. doi: 10.1080/15592324.2015.1049790. Plant Signal Behav. 2015. PMID: 26179579 Free PMC article.
-
Phosphatidic acid (PA) binds PP2AA1 to regulate PP2A activity and PIN1 polar localization.Mol Plant. 2013 Sep;6(5):1692-702. doi: 10.1093/mp/sst076. Epub 2013 May 17. Mol Plant. 2013. PMID: 23686948
-
Auxin gradients trigger de novo formation of stem cells during somatic embryogenesis.Plant Signal Behav. 2009 Jul;4(7):574-6. doi: 10.4161/psb.4.7.8730. Epub 2009 Jul 15. Plant Signal Behav. 2009. PMID: 19820347 Free PMC article. Review.
-
Subcellular trafficking of PIN auxin efflux carriers in auxin transport.Eur J Cell Biol. 2010 Feb-Mar;89(2-3):231-5. doi: 10.1016/j.ejcb.2009.11.003. Epub 2009 Nov 26. Eur J Cell Biol. 2010. PMID: 19944476 Review.
Cited by
-
Genome editing of a rice CDP-DAG synthase confers multipathogen resistance.Nature. 2023 Jun;618(7967):1017-1023. doi: 10.1038/s41586-023-06205-2. Epub 2023 Jun 14. Nature. 2023. PMID: 37316672 Free PMC article.
-
CDP-DAG synthases regulate plant growth and broad-spectrum disease resistance.Plant Signal Behav. 2025 Dec;20(1):2471503. doi: 10.1080/15592324.2025.2471503. Epub 2025 Feb 25. Plant Signal Behav. 2025. PMID: 39996429 Free PMC article.
-
Phosphatidic acid signaling in modulating plant reproduction and architecture.Plant Commun. 2025 Feb 10;6(2):101234. doi: 10.1016/j.xplc.2024.101234. Epub 2024 Dec 24. Plant Commun. 2025. PMID: 39722455 Free PMC article. Review.
-
Comprehensive metabolomic and lipidomic alterations in response to heat stress during seed germination and seedling growth of Arabidopsis.Front Plant Sci. 2023 Mar 29;14:1132881. doi: 10.3389/fpls.2023.1132881. eCollection 2023. Front Plant Sci. 2023. PMID: 37063208 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

