Motor defects in a Drosophila model for spinal muscular atrophy result from SMN depletion during early neurogenesis
- PMID: 35877682
- PMCID: PMC9352204
- DOI: 10.1371/journal.pgen.1010325
Motor defects in a Drosophila model for spinal muscular atrophy result from SMN depletion during early neurogenesis
Abstract
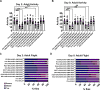
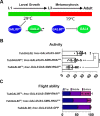
Spinal muscular atrophy (SMA) is the most common autosomal recessive neurodegenerative disease, and is characterised by spinal motor neuron loss, impaired motor function and, often, premature death. Mutations and deletions in the widely expressed survival motor neuron 1 (SMN1) gene cause SMA; however, the mechanisms underlying the selectivity of motor neuron degeneration are not well understood. Although SMA is degenerative in nature, SMN function during embryonic and early postnatal development appears to be essential for motor neuron survival in animal models and humans. Notwithstanding, how developmental defects contribute to the subversion of postnatal and adult motor function remains elusive. Here, in a Drosophila SMA model, we show that neurodevelopmental defects precede gross locomotor dysfunction in larvae. Furthermore, to specifically address the relevance of SMN during neurogenesis and in neurogenic cell types, we show that SMN knockdown using neuroblast-specific and pan-neuronal drivers, but not differentiated neuron or glial cell drivers, impairs adult motor function. Using targeted knockdown, we further restricted SMN manipulation in neuroblasts to a defined time window. Our aim was to express specifically in the neuronal progenitor cell types that have not formed synapses, and thus a time that precedes neuromuscular junction formation and maturation. By restoring SMN levels in these distinct neuronal population, we partially rescue the larval locomotor defects of Smn mutants. Finally, combinatorial SMN knockdown in immature and mature neurons synergistically enhances the locomotor and survival phenotypes. Our in-vivo study is the first to directly rescue the motor defects of an SMA model by expressing Smn in an identifiable population of Drosophila neuroblasts and developing neurons, highlighting that neuronal sensitivity to SMN loss may arise before synapse establishment and nerve cell maturation.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

