Antiviral Cyclopropane Acids from Deep-Sea-Derived Fungus Aspergillus sydowii
- PMID: 35877703
- PMCID: PMC9321810
- DOI: 10.3390/md20070410
Antiviral Cyclopropane Acids from Deep-Sea-Derived Fungus Aspergillus sydowii
Abstract
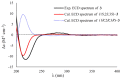
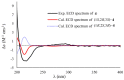
Four novel monocyclic cyclopropane acids, namely, sydocyclopropanes A-D (1-4), along with one known congener hamavellone B (5), were isolated from the Aspergillus sydowii MCCC 3A00324 fungus, which was isolated from the deep-sea sediment. The gross structures of novel compounds were established by detailed analyses of the spectroscopic data (HRESIMS and NMR spectra), and their absolute configurations were resolved on the basis of the quantum chemical calculations of ECD and NMR data, in association with DP4+ probability analyses. Sydocyclopropanes A-D, featuring the 1,1,2,3-tetrasubstituted cyclopropane nucleus with different lengthy alkyl side chains, were discovered in nature for the first time. All compounds exhibited antiviral activities against A/WSN/33 (H1N1), with IC50 values ranging from 26.7 to 77.2 μM, of which compound 1 exhibited a moderate inhibitory effect (IC50 = 26.7 μM).
Keywords: Aspergillus sydowii; H1N1; antiviral activities; cyclopropane; deep-sea-derived fungus.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

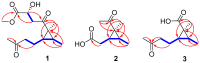
 ) and key HMBC (
) and key HMBC ( ) correlations of 1–3.
) correlations of 1–3.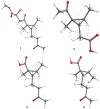
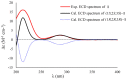
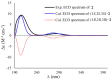
Similar articles
-
Secondary Metabolites, Biological Activities, and Industrial and Biotechnological Importance of Aspergillus sydowii.Mar Drugs. 2023 Aug 5;21(8):441. doi: 10.3390/md21080441. Mar Drugs. 2023. PMID: 37623723 Free PMC article. Review.
-
Phenolic bisabolane and cuparene sesquiterpenoids with anti-inflammatory activities from the deep-sea-derived Aspergillus sydowii MCCC 3A00324 fungus.Bioorg Chem. 2020 Dec;105:104420. doi: 10.1016/j.bioorg.2020.104420. Epub 2020 Oct 22. Bioorg Chem. 2020. PMID: 33152648
-
Acremolin D, a new acremolin alkaloid from the deep-sea sediment derived Aspergillus sydowii fungus.Nat Prod Res. 2022 Oct;36(19):4936-4942. doi: 10.1080/14786419.2021.1913587. Epub 2021 May 12. Nat Prod Res. 2022. PMID: 33977846
-
Structurally diverse sesquiterpenoids and polyketides from a sponge-associated fungus Aspergillus sydowii SCSIO41301.Fitoterapia. 2019 Jun;135:27-32. doi: 10.1016/j.fitote.2019.03.031. Epub 2019 Apr 1. Fitoterapia. 2019. PMID: 30946944
-
New Monoterpenoids and Polyketides from the Deep-Sea Sediment-Derived Fungus Aspergillus sydowii MCCC 3A00324.Mar Drugs. 2020 Nov 17;18(11):561. doi: 10.3390/md18110561. Mar Drugs. 2020. PMID: 33212800 Free PMC article.
Cited by
-
Phaeosphamides A and B, Cytotoxic Cyclodecadepsipeptides from the Mangrove-Derived Fungus Phaeosphaeriopsis sp. S296.Mar Drugs. 2022 Sep 21;20(10):591. doi: 10.3390/md20100591. Mar Drugs. 2022. PMID: 36286415 Free PMC article.
-
Secondary Metabolites, Biological Activities, and Industrial and Biotechnological Importance of Aspergillus sydowii.Mar Drugs. 2023 Aug 5;21(8):441. doi: 10.3390/md21080441. Mar Drugs. 2023. PMID: 37623723 Free PMC article. Review.
References
-
- Fan Y., Gao X., Yue J. Attractive natural products with strained cyclopropane and/or cyclobutane ring systems. Sci. China Chem. 2016;59:1126–1141. doi: 10.1007/s11426-016-0233-1. - DOI
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources

