An Update of Lectins from Marine Organisms: Characterization, Extraction Methodology, and Potential Biofunctional Applications
- PMID: 35877723
- PMCID: PMC9316650
- DOI: 10.3390/md20070430
An Update of Lectins from Marine Organisms: Characterization, Extraction Methodology, and Potential Biofunctional Applications
Abstract
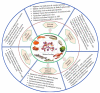
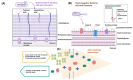
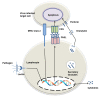
Lectins are a unique group of nonimmune carbohydrate-binding proteins or glycoproteins that exhibit specific and reversible carbohydrate-binding activity in a non-catalytic manner. Lectins have diverse sources and are classified according to their origins, such as plant lectins, animal lectins, and fish lectins. Marine organisms including fish, crustaceans, and mollusks produce a myriad of lectins, including rhamnose binding lectins (RBL), fucose-binding lectins (FTL), mannose-binding lectin, galectins, galactose binding lectins, and C-type lectins. The widely used method of extracting lectins from marine samples is a simple two-step process employing a polar salt solution and purification by column chromatography. Lectins exert several immunomodulatory functions, including pathogen recognition, inflammatory reactions, participating in various hemocyte functions (e.g., agglutination), phagocytic reactions, among others. Lectins can also control cell proliferation, protein folding, RNA splicing, and trafficking of molecules. Due to their reported biological and pharmaceutical activities, lectins have attracted the attention of scientists and industries (i.e., food, biomedical, and pharmaceutical industries). Therefore, this review aims to update current information on lectins from marine organisms, their characterization, extraction, and biofunctionalities.
Keywords: adhesins; bio-functional roles; characterization; extraction and purification; hemagglutinins; immunomodulation; marine lectins.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Kotecha H., Poduval P. Microbial lectins: Roles and applications. In: Surya M., Milind N., editors. Advances in Biological Science Research: A Practical Approach. Elsevier; Amsterdam, The Netherlands: 2019.
-
- Muramoto K. Lectins as Bioactive Proteins in Foods and Feeds. Food Sci. Technol. Res. 2017;23:487–494. doi: 10.3136/fstr.23.487. - DOI
-
- Elumalai P., Rubeena A.S., Arockiaraj J., Wongpanya R., Cammarata M., Ringø E., Vaseeharan B. The role of lectins in finfish: A review. Rev. Fish. Sci. Aquac. 2019;27:152–169. doi: 10.1080/23308249.2018.1520191. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

