IFN-γ-independent control of M. tuberculosis requires CD4 T cell-derived GM-CSF and activation of HIF-1α
- PMID: 35877763
- PMCID: PMC9352196
- DOI: 10.1371/journal.ppat.1010721
IFN-γ-independent control of M. tuberculosis requires CD4 T cell-derived GM-CSF and activation of HIF-1α
Abstract
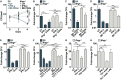
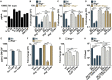
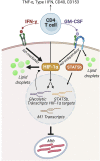
The prevailing model of protective immunity to tuberculosis is that CD4 T cells produce the cytokine IFN-γ to activate bactericidal mechanisms in infected macrophages. Although IFN-γ-independent CD4 T cell based control of M. tuberculosis infection has been demonstrated in vivo it is unclear whether CD4 T cells are capable of directly activating macrophages to control infection in the absence of IFN-γ. We developed a co-culture model using CD4 T cells isolated from the lungs of infected mice and M. tuberculosis-infected murine bone marrow-derived macrophages (BMDMs) to investigate mechanisms of CD4 dependent control of infection. We found that even in the absence of IFN-γ signaling, CD4 T cells drive macrophage activation, M1 polarization, and control of infection. This IFN-γ-independent control of infection requires activation of the transcription factor HIF-1α and a shift to aerobic glycolysis in infected macrophages. While HIF-1α activation following IFN-γ stimulation requires nitric oxide, HIF-1α-mediated control in the absence of IFN-γ is nitric oxide-independent, indicating that distinct pathways can activate HIF-1α during infection. We show that CD4 T cell-derived GM-CSF is required for IFN-γ-independent control in BMDMs, but that recombinant GM-CSF is insufficient to control infection in BMDMs or alveolar macrophages and does not rescue the absence of control by GM-CSF-deficient T cells. In contrast, recombinant GM-CSF controls infection in peritoneal macrophages, induces lipid droplet biogenesis, and also requires HIF-1α for control. These results advance our understanding of CD4 T cell-mediated immunity to M. tuberculosis, reveal important differences in immune activation of distinct macrophage types, and outline a novel mechanism for the activation of HIF-1α. We establish a previously unknown functional link between GM-CSF and HIF-1α and provide evidence that CD4 T cell-derived GM-CSF is a potent bactericidal effector.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

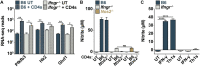


Similar articles
-
Role of Granulocyte-Macrophage Colony-Stimulating Factor Production by T Cells during Mycobacterium tuberculosis Infection.mBio. 2017 Oct 24;8(5):e01514-17. doi: 10.1128/mBio.01514-17. mBio. 2017. PMID: 29066547 Free PMC article.
-
HIF-1α Is an Essential Mediator of IFN-γ-Dependent Immunity to Mycobacterium tuberculosis.J Immunol. 2016 Aug 15;197(4):1287-97. doi: 10.4049/jimmunol.1600266. Epub 2016 Jul 18. J Immunol. 2016. PMID: 27430718 Free PMC article.
-
Nitric Oxide Modulates Macrophage Responses to Mycobacterium tuberculosis Infection through Activation of HIF-1α and Repression of NF-κB.J Immunol. 2017 Sep 1;199(5):1805-1816. doi: 10.4049/jimmunol.1700515. Epub 2017 Jul 28. J Immunol. 2017. PMID: 28754681 Free PMC article.
-
In vitro induction of inhibitory macrophage differentiation by granulocyte-macrophage colony-stimulating factor, stem cell factor and interferon-gamma from lineage phenotypes-negative c-kit-positive murine hematopoietic progenitor cells.Immunol Lett. 2004 Feb 15;91(2-3):221-7. doi: 10.1016/j.imlet.2003.12.008. Immunol Lett. 2004. PMID: 15019293
-
Relationship between granulocyte macrophage-colony stimulating factor, tumour necrosis factor-alpha and Trypanosoma cruzi infection of murine macrophages.Parasite Immunol. 1995 Mar;17(3):135-41. doi: 10.1111/j.1365-3024.1995.tb01015.x. Parasite Immunol. 1995. PMID: 7792097
Cited by
-
Tuberculous Meningitis Genetic predisposition: Understanding cellular interactions, molecular mechanisms and genetic dimensions.Tunis Med. 2024 Aug 5;102(8):440-446. doi: 10.62438/tunismed.v102i8.4816. Tunis Med. 2024. PMID: 39129569 Free PMC article. Review. French.
-
Transcriptional analysis of human peripheral blood mononuclear cells stimulated by Mycobacterium tuberculosis antigen.Front Cell Infect Microbiol. 2023 Sep 25;13:1255905. doi: 10.3389/fcimb.2023.1255905. eCollection 2023. Front Cell Infect Microbiol. 2023. PMID: 37818041 Free PMC article.
-
Re-appraising the role of T-cell derived interferon gamma in restriction of Mycobacterium tuberculosis in the murine lung: T-cell derived IFNγ is required to restrict pulmonary Mtb.bioRxiv [Preprint]. 2024 Apr 5:2024.04.04.588086. doi: 10.1101/2024.04.04.588086. bioRxiv. 2024. Update in: J Immunol. 2024 Aug 1;213(3):339-346. doi: 10.4049/jimmunol.2400145. PMID: 38617280 Free PMC article. Updated. Preprint.
-
Angiogenesis during diabetic wound repair: from mechanism to therapy opportunity.Burns Trauma. 2025 Feb 7;13:tkae052. doi: 10.1093/burnst/tkae052. eCollection 2025. Burns Trauma. 2025. PMID: 39927093 Free PMC article. Review.
-
Tax1bp1 enhances bacterial virulence and promotes inflammatory responses during Mycobacterium tuberculosis infection of alveolar macrophages.bioRxiv [Preprint]. 2024 Dec 16:2024.12.16.628616. doi: 10.1101/2024.12.16.628616. bioRxiv. 2024. PMID: 39763950 Free PMC article. Preprint.
References
-
- Flesch IE, Kaufmann SH. Mycobacterial growth inhibition by interferon-gamma-activated bone marrow macrophages and differential susceptibility among strains of Mycobacterium tuberculosis. Journal of immunology (Baltimore, Md: 1950). 1987;138(12). - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

