Astragalus polysaccharides and astragaloside IV alleviate inflammation in bovine mammary epithelial cells by regulating Wnt/β-catenin signaling pathway
- PMID: 35877777
- PMCID: PMC9312414
- DOI: 10.1371/journal.pone.0271598
Astragalus polysaccharides and astragaloside IV alleviate inflammation in bovine mammary epithelial cells by regulating Wnt/β-catenin signaling pathway
Abstract
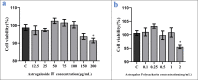
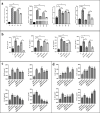
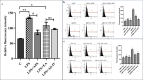
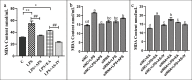
The Wnt/β-catenin signaling regulates cell renewal and repair and is closely associated with inflammation. Astragalus polysaccharides (APS) and astragaloside IV (AS-IV), which are the main active substances extracted from Radix Astragali, protect cells by regulating Wnt signaling in cells, exerting antiinflammatory, antioxidant, and antistress effects. However, the mechanisms by which APS and AS-IV interact with Wnt signaling to achieve their therapeutic effects in bovine mammary epithelial cells (BMECs) are not understood. In this study, we used lipopolysaccharide (LPS)-stimulated BMECs as an in vitro model of inflammation to investigate the effects of APS and AS-IV on Wnt signaling in inflamed BMECs. Drug concentrations were screened using the CCK-8 method, the effect on protein expression was analyzed using immunoblotting, the effect on inflammatory factors using enzyme-linked immunosorbent assay, and the effect on oxidative factors using enzyme labeling and flow cytometry. LPS activated the expression of inflammatory and oxidative factors in cells and inhibited Wnt/β-catenin signaling. APS and AS-IV antagonized the inhibitory effect of LPS, protecting BMECs. They inhibited the expression of the IL-6, IL-8, and TNF-α inflammatory factors, and that of the MDA oxidative factor, and activated Wnt signaling in LPS-stimulated BMECs. Silencing of β-catenin abolished the protective effect of APS and AS-IV against LPS-stimulated BMECs. Thus, APS and AS-IV mediate protective effects in inflammatory BMECs model through activation of the Wnt signaling pathway. Wnt signaling pathway is one of the targets of the inhibitory effects of APS and AS-IV on inflammation.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures



Similar articles
-
Astragalus polysaccharides alleviates LPS-induced inflammation via the NF-κB/MAPK signaling pathway.J Cell Physiol. 2020 Jul;235(7-8):5525-5540. doi: 10.1002/jcp.29452. Epub 2020 Feb 9. J Cell Physiol. 2020. PMID: 32037545
-
Protective Effects of Astragalus Polysaccharide on Sepsis-Induced Acute Kidney Injury.Anal Cell Pathol (Amst). 2021 Jan 26;2021:7178253. doi: 10.1155/2021/7178253. eCollection 2021. Anal Cell Pathol (Amst). 2021. PMID: 33575163 Free PMC article.
-
Astragalus polysaccharide inhibits breast cancer cell migration and invasion by regulating epithelial‑mesenchymal transition via the Wnt/β‑catenin signaling pathway.Mol Med Rep. 2020 Apr;21(4):1819-1832. doi: 10.3892/mmr.2020.10983. Epub 2020 Feb 12. Mol Med Rep. 2020. PMID: 32319619 Free PMC article.
-
Astragaloside IV derived from Astragalus membranaceus: A research review on the pharmacological effects.Adv Pharmacol. 2020;87:89-112. doi: 10.1016/bs.apha.2019.08.002. Epub 2019 Dec 18. Adv Pharmacol. 2020. PMID: 32089240 Review.
-
Fufang shenhua tablet, astragali radix and its active component astragaloside IV: Research progress on anti-inflammatory and immunomodulatory mechanisms in the kidney.Front Pharmacol. 2023 Apr 5;14:1131635. doi: 10.3389/fphar.2023.1131635. eCollection 2023. Front Pharmacol. 2023. PMID: 37089929 Free PMC article. Review.
Cited by
-
Research state of the herbal medicine Huangqi (Radix Astragali): A global and bibliometric study.Medicine (Baltimore). 2024 Feb 23;103(8):e37277. doi: 10.1097/MD.0000000000037277. Medicine (Baltimore). 2024. PMID: 38394541 Free PMC article.
-
Success and Controversy of Natural Products as Therapeutic Modulators of Wnt Signaling and Its Interplay with Oxidative Stress: Comprehensive Review Across Compound Classes and Experimental Systems.Antioxidants (Basel). 2025 May 14;14(5):591. doi: 10.3390/antiox14050591. Antioxidants (Basel). 2025. PMID: 40427472 Free PMC article. Review.
-
Astragali radix (Huangqi): a time-honored nourishing herbal medicine.Chin Med. 2024 Aug 30;19(1):119. doi: 10.1186/s13020-024-00977-z. Chin Med. 2024. PMID: 39215362 Free PMC article. Review.
-
The Large Molecular Weight Polysaccharide from Wild Cordyceps and Its Antitumor Activity on H22 Tumor-Bearing Mice.Molecules. 2023 Apr 10;28(8):3351. doi: 10.3390/molecules28083351. Molecules. 2023. PMID: 37110586 Free PMC article.
-
Huangqi fuling decoction inhibits the invasion and metastasis of gastric cancer via the TNF signaling pathway.Sci Rep. 2025 Jun 4;15(1):19628. doi: 10.1038/s41598-025-00920-8. Sci Rep. 2025. PMID: 40467576 Free PMC article.
References
-
- Sun Y, Li L, Wu J, Yu P, Li C, Tang J, et al.. Bovine recombinant lipopolysaccharide binding protein (BRLBP) regulated apoptosis and inflammation response in lipopolysaccharide-challenged bovine mammary epithelial cells (BMEC)[J]. Molecular Immunology, 2015,65(2):205–214. doi: 10.1016/j.molimm.2015.01.026 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

