Dual-Phase Mixed Protonic-Electronic Conducting Hydrogen Separation Membranes: A Review
- PMID: 35877850
- PMCID: PMC9320335
- DOI: 10.3390/membranes12070647
Dual-Phase Mixed Protonic-Electronic Conducting Hydrogen Separation Membranes: A Review
Abstract
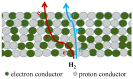
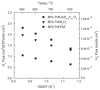
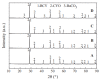
Owing to the excellent properties of high selectivity, high thermal stability, and low cost, in the past twenty years, mixed protonic-electronic conducting hydrogen separation membranes have received extensive attention. In particular, dual-phase mixed protonic-electronic conducting membranes with high ambipolar conductivity are more attractive because of the high hydrogen permeability. This paper aimed to present a review of research activities on the dual-phase membranes, in which the components, the characteristics, and the performances of different dual-phase membranes are introduced. The key issues that affect the membrane performance such as the elimination of the inter-phase reaction, the combination mode of the phases, the phase ratio, and the membrane configuration were discussed. The current problems and future trends were simply recommended.
Keywords: dual-phase; hydrogen separation; membrane; mixed protonic-electronic conducting.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







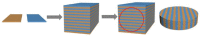

Similar articles
-
Design of Mixed Ionic-Electronic Materials for Permselective Membranes and Solid Oxide Fuel Cells Based on Their Oxygen and Hydrogen Mobility.Membranes (Basel). 2023 Jul 27;13(8):698. doi: 10.3390/membranes13080698. Membranes (Basel). 2023. PMID: 37623759 Free PMC article. Review.
-
Machine-Learning-Accelerated Development of Efficient Mixed Protonic-Electronic Conducting Oxides as the Air Electrodes for Protonic Ceramic Cells.Adv Mater. 2022 Dec;34(51):e2203446. doi: 10.1002/adma.202203446. Epub 2022 Nov 14. Adv Mater. 2022. PMID: 36177694
-
Thermodynamic Insights for Electrochemical Hydrogen Compression with Proton-Conducting Membranes.Membranes (Basel). 2019 Jul 1;9(7):77. doi: 10.3390/membranes9070077. Membranes (Basel). 2019. PMID: 31266218 Free PMC article.
-
Investigation of La1-xSrxCrO3-∂ (x ~ 0.1) as Membrane for Hydrogen Production.Membranes (Basel). 2012 Sep 11;2(3):665-86. doi: 10.3390/membranes2030665. Membranes (Basel). 2012. PMID: 24958299 Free PMC article.
-
Microstructural and Interfacial Designs of Oxygen-Permeable Membranes for Oxygen Separation and Reaction-Separation Coupling.Adv Mater. 2019 Dec;31(50):e1902547. doi: 10.1002/adma.201902547. Epub 2019 Aug 16. Adv Mater. 2019. PMID: 31418945 Review.
Cited by
-
Optimization of Carbon Dioxide Utilization: Simulation-Based Analysis of Reverse Water Gas Shift Membrane Reactors.Membranes (Basel). 2025 Apr 1;15(4):107. doi: 10.3390/membranes15040107. Membranes (Basel). 2025. PMID: 40277978 Free PMC article.
-
Design and Validation of an Experimental Setup for Evaluation of Gas Permeation in Ceramic Membranes.Membranes (Basel). 2023 Feb 18;13(2):246. doi: 10.3390/membranes13020246. Membranes (Basel). 2023. PMID: 36837749 Free PMC article.
-
Structural and Transport Properties of E-Beam Sintered Lanthanide Tungstates and Tungstates-Molybdates.Nanomaterials (Basel). 2022 Sep 21;12(19):3282. doi: 10.3390/nano12193282. Nanomaterials (Basel). 2022. PMID: 36234410 Free PMC article.
-
Design of Mixed Ionic-Electronic Materials for Permselective Membranes and Solid Oxide Fuel Cells Based on Their Oxygen and Hydrogen Mobility.Membranes (Basel). 2023 Jul 27;13(8):698. doi: 10.3390/membranes13080698. Membranes (Basel). 2023. PMID: 37623759 Free PMC article. Review.
-
Synthesis and Oxygen Mobility of Bismuth Cerates and Titanates with Pyrochlore Structure.Membranes (Basel). 2023 Jun 13;13(6):598. doi: 10.3390/membranes13060598. Membranes (Basel). 2023. PMID: 37367802 Free PMC article.
References
-
- Yang M., He F., Zhou C., Dong F., Yang G., Zhou W., Shao Z. New perovskite membrane with improved sintering and self-reconstructed surface for efficient hydrogen permeation. J. Memb. Sci. 2021;620:118980. doi: 10.1016/j.memsci.2020.118980. - DOI
-
- Saini N., Awasthi K. Insights into the progress of polymeric nano-composite membranes for hydrogen separation and purification in the direction of sustainable energy resources. Sep. Purif. Technol. 2022;282:120029. doi: 10.1016/j.seppur.2021.120029. - DOI
-
- Pal N., Agarwal M. Advances in materials process and separation mechanism of the membrane towards hydrogen separation. Int. J. Hydrogen Energy. 2021;46:27062–27087. doi: 10.1016/j.ijhydene.2021.05.175. - DOI
-
- Winter C.J. Hydrogen energy-Abundant, efficient, clean: A debate over the energy-system-of-change. Int. J. Hydrogen Energy. 2009;34:S1–S52. doi: 10.1016/j.ijhydene.2009.05.063. - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

