The Short-Term Opening of Cyclosporin A-Independent Palmitate/Sr2+-Induced Pore Can Underlie Ion Efflux in the Oscillatory Mode of Functioning of Rat Liver Mitochondria
- PMID: 35877870
- PMCID: PMC9319229
- DOI: 10.3390/membranes12070667
The Short-Term Opening of Cyclosporin A-Independent Palmitate/Sr2+-Induced Pore Can Underlie Ion Efflux in the Oscillatory Mode of Functioning of Rat Liver Mitochondria
Abstract
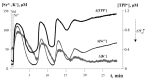
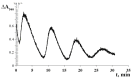
Mitochondria are capable of synchronized oscillations in many variables, but the underlying mechanisms are still unclear. In this study, we demonstrated that rat liver mitochondria, when exposed to a pulse of Sr2+ ions in the presence of valinomycin (a potassium ionophore) and cyclosporin A (a specific inhibitor of the permeability transition pore complex) under hypotonia, showed prolonged oscillations in K+ and Sr2+ fluxes, membrane potential, pH, matrix volume, rates of oxygen consumption and H2O2 formation. The dynamic changes in the rate of H2O2 production were in a reciprocal relationship with the respiration rate and in a direct relationship with the mitochondrial membrane potential and other indicators studied. The pre-incubation of mitochondria with Ca2+(Sr2+)-dependent phospholipase A2 inhibitors considerably suppressed the accumulation of free fatty acids, including palmitic and stearic acids, and all spontaneous Sr2+-induced cyclic changes. These data suggest that the mechanism of ion efflux from mitochondria is related to the opening of short-living pores, which can be caused by the formation of complexes between Sr2+(Ca2+) and endogenous long-chain saturated fatty acids (mainly, palmitic acid) that accumulate due to the activation of phospholipase A2 by the ions. A possible role for transient palmitate/Ca2+(Sr2+)-induced pores in the maintenance of ion homeostasis and the prevention of calcium overload in mitochondria under pathophysiological conditions is discussed.
Keywords: cyclosporin A; cyclosporin A-independent palmitate/Ca2+-induced permeability transition pore; ion oscillations; lipid pore; mitochondria; mitochondrial permeability transition; palmitic acid; phospholipase A2.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
Involvement of palmitate/Ca2+(Sr2+)-induced pore in the cycling of ions across the mitochondrial membrane.Biochim Biophys Acta. 2015 Feb;1848(2):488-95. doi: 10.1016/j.bbamem.2014.10.027. Epub 2014 Oct 25. Biochim Biophys Acta. 2015. PMID: 25450352
-
Inhibition of the mitochondrial permeability transition by cyclosporin A during long time frame experiments: relationship between pore opening and the activity of mitochondrial phospholipases.Biochemistry. 1995 Dec 19;34(50):16440-9. doi: 10.1021/bi00050a027. Biochemistry. 1995. PMID: 8845372
-
Mitochondrial Ca2+ cycle mediated by the palmitate-activated cyclosporin A-insensitive pore.J Bioenerg Biomembr. 2007 Apr;39(2):167-74. doi: 10.1007/s10863-007-9079-9. Epub 2007 May 25. J Bioenerg Biomembr. 2007. PMID: 17530392
-
Mitochondrial Cyclosporine A-Independent Palmitate/Ca2+-Induced Permeability Transition Pore (PA-mPT Pore) and Its Role in Mitochondrial Function and Protection against Calcium Overload and Glutamate Toxicity.Cells. 2021 Jan 11;10(1):125. doi: 10.3390/cells10010125. Cells. 2021. PMID: 33440765 Free PMC article. Review.
-
Sustained oscillations of transmembrane Ca2+ fluxes in mitochondria and their possible biological significance.Membr Cell Biol. 2000;14(1):1-17. Membr Cell Biol. 2000. PMID: 11051078 Review.
Cited by
-
Protective Effect of Uridine on Structural and Functional Rearrangements in Heart Mitochondria after a High-Dose Isoprenaline Exposure Modelling Stress-Induced Cardiomyopathy in Rats.Int J Mol Sci. 2023 Dec 9;24(24):17300. doi: 10.3390/ijms242417300. Int J Mol Sci. 2023. PMID: 38139129 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

