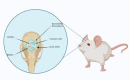
Small Intestinal Submucosa Biomimetic Periosteum Promotes Bone Regeneration
- PMID: 35877922
- PMCID: PMC9323854
- DOI: 10.3390/membranes12070719
Small Intestinal Submucosa Biomimetic Periosteum Promotes Bone Regeneration
Abstract
Background: Critical bone defects are a significant problem in clinics. The periosteum plays a vital role in bone regeneration. A tissue-engineered periosteum (TEP) has received increasing attention as a novel strategy for bone defect repairs.
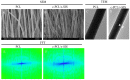
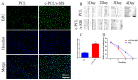
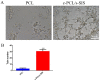
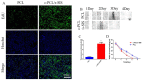
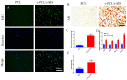
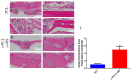
Methods: In this experiment, a biomimetic periosteum was fabricated by using coaxial electrospinning technology with decellularized porcine small intestinal submucosa (SIS) as the shell and polycaprolactone (PCL) as the core. In vitro, the effects of the biomimetic periosteum on Schwann cells, vascular endothelial cells, and bone marrow mesenchymal stem cells were detected by a scratch test, an EdU, a tube-forming test, and an osteogenesis test. In vivo, we used HE staining to evaluate the effect of the biomimetic periosteum on bone regeneration.
Results: In vitro experiments showed that the biomimetic periosteum could significantly promote the formation of angiogenesis, osteogenesis, and repaired Schwann cells (SCs). In vivo experiments showed that the biomimetic periosteum could promote the repair of bone defects.
Conclusions: The biomimetic periosteum could simulate the structural function of the periosteum and promote bone repair. This strategy may provide a promising method for the clinical treatment of skull bone defects.
Keywords: Schwann cells; angiogenesis; biomimetic periosteum; bone regeneration; ordered coaxial electrospinning.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Koons G.L., Diba M., Mikos A.G. Materials design for bone-tissue engineering. Nat. Rev. Mater. 2020;5:584–603. doi: 10.1038/s41578-020-0204-2. - DOI
LinkOut - more resources
Full Text Sources

