Molecular determinants of pH sensing in the proton-activated chloride channel
- PMID: 35878032
- PMCID: PMC9351481
- DOI: 10.1073/pnas.2200727119
Molecular determinants of pH sensing in the proton-activated chloride channel
Abstract
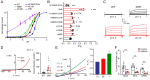
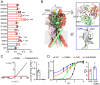
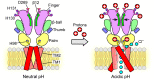
In response to acidic pH, the widely expressed proton-activated chloride (PAC) channel opens and conducts anions across cellular membranes. By doing so, PAC plays an important role in both cellular physiology (endosome acidification) and diseases associated with tissue acidosis (acid-induced cell death). Despite the available structural information, how proton binding in the extracellular domain (ECD) leads to PAC channel opening remains largely unknown. Here, through comprehensive mutagenesis and electrophysiological studies, we identified several critical titratable residues, including two histidine residues (H130 and H131) and an aspartic acid residue (D269) at the distal end of the ECD, together with the previously characterized H98 at the transmembrane domain-ECD interface, as potential pH sensors for human PAC. Mutations of these residues resulted in significant changes in pH sensitivity. Some combined mutants also exhibited large basal PAC channel activities at neutral pH. By combining molecular dynamics simulations with structural and functional analysis, we further found that the β12 strand at the intersubunit interface and the associated "joint region" connecting the upper and lower ECDs allosterically regulate the proton-dependent PAC activation. Our studies suggest a distinct pH-sensing and gating mechanism of this new family of ion channels sensitive to acidic environment.
Keywords: PAC; TMEM206; allosteric regulation; pH sensitivity; proton-activated chloride channel.
Conflict of interest statement
The authors declare no competing interest.
Figures




Similar articles
-
Molecular mechanism underlying desensitization of the proton-activated chloride channel PAC.Elife. 2022 Dec 22;11:e82955. doi: 10.7554/eLife.82955. Elife. 2022. PMID: 36547405 Free PMC article.
-
Identification of the Acid-Sensitive Site Critical for Chloral Hydrate (CH) Activation of the Proton-Activated Chloride Channel.J Neurosci. 2023 Jan 25;43(4):526-539. doi: 10.1523/JNEUROSCI.0482-22.2022. Epub 2022 Oct 25. J Neurosci. 2023. PMID: 36283831 Free PMC article.
-
Structures and pH-sensing mechanism of the proton-activated chloride channel.Nature. 2020 Dec;588(7837):350-354. doi: 10.1038/s41586-020-2875-7. Epub 2020 Nov 4. Nature. 2020. PMID: 33149300 Free PMC article.
-
Proton-Activated Chloride Channel: Physiology and Disease.Front Biosci (Landmark Ed). 2023 Jan 16;28(1):11. doi: 10.31083/j.fbl2801011. Front Biosci (Landmark Ed). 2023. PMID: 36722267 Review.
-
Emerging issues of connexin channels: biophysics fills the gap.Q Rev Biophys. 2001 Aug;34(3):325-472. doi: 10.1017/s0033583501003705. Q Rev Biophys. 2001. PMID: 11838236 Review.
Cited by
-
A new polymodal gating model of the proton-activated chloride channel.PLoS Biol. 2023 Sep 15;21(9):e3002309. doi: 10.1371/journal.pbio.3002309. eCollection 2023 Sep. PLoS Biol. 2023. PMID: 37713449 Free PMC article.
-
Inhibition of the proton-activated chloride channel PAC by PIP2.Elife. 2023 Jan 12;12:e83935. doi: 10.7554/eLife.83935. Elife. 2023. PMID: 36633397 Free PMC article.
-
Constant pH molecular dynamics simulations: Current status and recent applications.Curr Opin Struct Biol. 2022 Dec;77:102498. doi: 10.1016/j.sbi.2022.102498. Epub 2022 Nov 18. Curr Opin Struct Biol. 2022. PMID: 36410222 Free PMC article. Review.
-
Weak Acids as Endogenous Inhibitors of the Proton-Activated Chloride Channel.Cells. 2025 Jul 19;14(14):1110. doi: 10.3390/cells14141110. Cells. 2025. PMID: 40710363 Free PMC article.
-
Bicarbonate signalling via G protein-coupled receptor regulates ischaemia-reperfusion injury.Nat Commun. 2024 Feb 27;15(1):1530. doi: 10.1038/s41467-024-45579-3. Nat Commun. 2024. PMID: 38413581 Free PMC article.
References
-
- Capurro V., et al. , Functional analysis of acid-activated Cl− channels: Properties and mechanisms of regulation. Biochim. Biophys. Acta 1848, 105–114 (2015). - PubMed
-
- Auzanneau C., Thoreau V., Kitzis A., Becq F., A novel voltage-dependent chloride current activated by extracellular acidic pH in cultured rat Sertoli cells. J. Biol. Chem. 278, 19230–19236 (2003). - PubMed
-
- Sato-Numata K., Numata T., Okada T., Okada Y., Acid-sensitive outwardly rectifying (ASOR) anion channels in human epithelial cells are highly sensitive to temperature and independent of ClC-3. Pflugers Arch. 465, 1535–1543 (2013). - PubMed
-
- Ma Z. Y., et al. , A proton-activated, outwardly rectifying chloride channel in human umbilical vein endothelial cells. Biochem. Biophys. Res. Commun. 371, 437–440 (2008). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

