The role of prediction in learned predictiveness
- PMID: 35878082
- PMCID: PMC9310352
- DOI: 10.1037/xan0000330
The role of prediction in learned predictiveness
Abstract
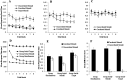
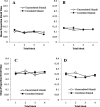
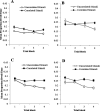
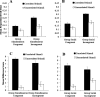
Learning permits even relatively uninteresting stimuli to capture attention if they are established as predictors of important outcomes. Associative theories explain this "learned predictiveness" effect by positing that attention is a function of the relative strength of the association between stimuli and outcomes. In three experiments we show that this explanation is incomplete: learned overt visual-attention is not a function of the relative strength of the association between stimuli and an outcome. In three experiments, human participants were exposed to triplets of stimuli that comprised (a) a target (that defined correct responding), (b) a stimulus that was perfectly correlated with the presentation of the target, and (c) a stimulus that was uncorrelated with the presentation of the target. Participants' knowledge of the associative relationship between the correlated or uncorrelated stimuli and the target was always good. However, eye-tracking revealed that an attentional bias toward the correlated stimulus only developed when it and target-relevant responding preceded the target stimulus. We propose a framework in which attentional changes are modulated during learning as a function the relative strength of the association between stimuli and the task-relevant response, rather than an association between stimuli and the task-relevant outcome. (PsycInfo Database Record (c) 2022 APA, all rights reserved).
Figures



Similar articles
-
The role of selection history in the learned predictiveness effect.J Exp Psychol Hum Percept Perform. 2024 Oct;50(10):1010-1022. doi: 10.1037/xhp0001240. Epub 2024 Aug 22. J Exp Psychol Hum Percept Perform. 2024. PMID: 39172367
-
Attention and associative learning in humans: An integrative review.Psychol Bull. 2016 Oct;142(10):1111-1140. doi: 10.1037/bul0000064. Epub 2016 Aug 8. Psychol Bull. 2016. PMID: 27504933 Review.
-
Prediction and uncertainty in associative learning: examining controlled and automatic components of learned attentional biases.Q J Exp Psychol (Hove). 2017 Aug;70(8):1485-1503. doi: 10.1080/17470218.2016.1188407. Epub 2016 Jun 7. Q J Exp Psychol (Hove). 2017. PMID: 27174735
-
The outcome predictability bias is evident in overt attention.J Exp Psychol Anim Learn Cogn. 2019 Jul;45(3):290-300. doi: 10.1037/xan0000210. Epub 2019 May 9. J Exp Psychol Anim Learn Cogn. 2019. PMID: 31070432
-
Attentional processes during emotional face perception in social anxiety disorder: A systematic review and meta-analysis of eye-tracking findings.Prog Neuropsychopharmacol Biol Psychiatry. 2021 Dec 20;111:110353. doi: 10.1016/j.pnpbp.2021.110353. Epub 2021 May 15. Prog Neuropsychopharmacol Biol Psychiatry. 2021. PMID: 34000291
References
-
- Allport A. (1989). Visual attention. In Posner M. I. (Ed.), Foundations of cognitive science (pp. 631–682). MIT Press.

