False Positivity of Anti-SARS-CoV-2 Antibodies in Patients with Acute Tropical Diseases in Thailand
- PMID: 35878144
- PMCID: PMC9320684
- DOI: 10.3390/tropicalmed7070132
False Positivity of Anti-SARS-CoV-2 Antibodies in Patients with Acute Tropical Diseases in Thailand
Abstract
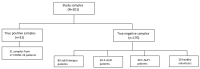
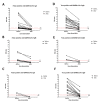
Serology remains a useful indirect method of diagnosing tropical diseases, especially in dengue infection. However, the current literature regarding cross-reactivity between SARS-CoV-2 and dengue serology is limited and revealed conflicting results. As a means to uncover relevant serological insight involving antibody classes against SARS-CoV-2 and cross-reactivity, anti-SARS-CoV-2 IgA, IgM, and IgG ELISA, based on spike and nucleocapsid proteins, were selected for a fever-presenting tropical disease patient investigation. The study was conducted at the Faculty of Tropical Medicine during March to December 2021. The study data source comprised (i) 170 non-COVID-19 sera from 140 adults and children presenting with acute undifferentiated febrile illness and 30 healthy volunteers, and (ii) 31 COVID-19 sera from 17 RT-PCR-confirmed COVID-19 patients. Among 170 non-COVID-19 samples, 27 were false positives (15.9%), of which IgA, IgM, and IgG cross-reactive antibody classes were detected in 18 (10.6%), 9 (5.3%), and 3 (1.8%) cases, respectively. Interestingly, one case exhibited both IgA and IgM false positivity, while two cases exhibited both IgA and IgG false positivity. The false positivity rate in anti-SARS-CoV-2 IgA and IgM was reported in adults with dengue infection (11.3% and 5%) and adults with other tropical diseases (16.7% and 13.3%). The urea dissociation method applied to mitigate false positivity resulted in significantly decreased ELISA-based false and true positives. In conclusion, the analysis of antibody against SARS-CoV-2 in sera of patients with different tropical diseases showed that high IgA and IgM false positivity thus potentially limits serological assay utility in fever-presenting patients in tropical areas.
Keywords: COVID-19; ELISA; SARS-CoV-2; Thailand; acute febrile illness; antibodies; cross reaction; dengue; false positive reaction; tropical diseases.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Longitudinal analysis to characterize classes and subclasses of antibody responses to recombinant receptor-binding protein (RBD) of SARS-CoV-2 in COVID-19 patients in Thailand.PLoS One. 2021 Aug 10;16(8):e0255796. doi: 10.1371/journal.pone.0255796. eCollection 2021. PLoS One. 2021. PMID: 34375345 Free PMC article.
-
Anti-Arbovirus Antibodies Cross-React With Severe Acute Respiratory Syndrome Coronavirus 2.Microbiol Spectr. 2022 Dec 21;10(6):e0263922. doi: 10.1128/spectrum.02639-22. Epub 2022 Nov 29. Microbiol Spectr. 2022. PMID: 36445096 Free PMC article.
-
Potential Antigenic Cross-reactivity Between Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) and Dengue Viruses.Clin Infect Dis. 2021 Oct 5;73(7):e2444-e2449. doi: 10.1093/cid/ciaa1207. Clin Infect Dis. 2021. PMID: 32797228 Free PMC article.
-
Diagnostic performance of four SARS-CoV-2 antibody assays in patients with COVID-19 or with bacterial and non-SARS-CoV-2 viral respiratory infections.Eur J Clin Microbiol Infect Dis. 2021 Sep;40(9):1983-1997. doi: 10.1007/s10096-021-04285-4. Epub 2021 Jun 9. Eur J Clin Microbiol Infect Dis. 2021. PMID: 34109500 Free PMC article.
-
Combined anti-SARS-CoV-2 IgA, IgG, and IgM Detection as a Better Strategy to Prevent Second Infection Spreading Waves.Immunol Invest. 2022 Feb;51(2):233-245. doi: 10.1080/08820139.2020.1823407. Epub 2020 Sep 18. Immunol Invest. 2022. PMID: 32945214 Free PMC article.
Cited by
-
Global Coinfections with Bacteria, Fungi, and Respiratory Viruses in Children with SARS-CoV-2: A Systematic Review and Meta-Analysis.Trop Med Infect Dis. 2022 Nov 15;7(11):380. doi: 10.3390/tropicalmed7110380. Trop Med Infect Dis. 2022. PMID: 36422931 Free PMC article. Review.
-
Effects of Recent Prior Dengue Infection on Risk and Severity of Subsequent SARS-CoV-2 Infection: A Retrospective Cohort Study.Open Forum Infect Dis. 2024 Jul 13;11(8):ofae397. doi: 10.1093/ofid/ofae397. eCollection 2024 Aug. Open Forum Infect Dis. 2024. PMID: 39091642 Free PMC article.
-
Assessment of Broadly Reactive Responses in Patients With MERS-CoV Infection and SARS-CoV-2 Vaccination.JAMA Netw Open. 2023 Jun 1;6(6):e2319222. doi: 10.1001/jamanetworkopen.2023.19222. JAMA Netw Open. 2023. PMID: 37389876 Free PMC article.
-
The Role of NS1 Protein in the Diagnosis of Flavivirus Infections.Viruses. 2023 Feb 19;15(2):572. doi: 10.3390/v15020572. Viruses. 2023. PMID: 36851784 Free PMC article. Review.
-
A novel highly specific biotinylated MAC-ELISA for detection of anti-SARS-CoV-2 nucleocapsid antigen IgM antibodies during the acute phase of COVID-19.Braz J Microbiol. 2023 Dec;54(4):2893-2901. doi: 10.1007/s42770-023-01160-6. Epub 2023 Nov 6. Braz J Microbiol. 2023. PMID: 37930615 Free PMC article.
References
-
- Zhu N., Zhang D., Wang W., Li X., Yang B., Song J., Zhao X., Huang B., Shi W., Lu R., et al. The China Novel Coronavirus Investigating and Research Team. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N. Engl. J. Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. - DOI - PMC - PubMed
-
- Ong D.S.Y., Fragkou P.C., Schweitzer V.A., Chemaly R.F., Moschopoulos C.D., Skevaki C. European Society of Clinical Microbiology and Infectious Diseases (ESCMID) Study Group for Respiratory Viruses (ESGREV). How to interpret and use COVID-19 serology and immunology tests. Clin. Microbiol. Infect. 2021;27:981–986. doi: 10.1016/j.cmi.2021.05.001. - DOI - PMC - PubMed
-
- Lutalo T., Nalumansi A., Olara D., Kayiwa J., Ogwang B., Odwilo E., Watera C., Balinandi S., Kiconco J., Nakaseegu J., et al. Evaluation of the performance of 25 SARS-CoV-2 serological rapid diagnostic tests using a reference panel of plasma specimens at the Uganda Virus Research Institute. Int. J. Infect. Dis. 2021;112:281–287. doi: 10.1016/j.ijid.2021.09.020. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

