Patulin Detoxification by Recombinant Manganese Peroxidase from Moniliophthora roreri Expressed by Pichia pastoris
- PMID: 35878178
- PMCID: PMC9324453
- DOI: 10.3390/toxins14070440
Patulin Detoxification by Recombinant Manganese Peroxidase from Moniliophthora roreri Expressed by Pichia pastoris
Abstract
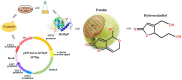
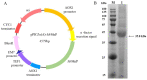
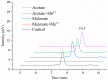
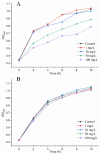
The fungal secondary metabolite patulin is a mycotoxin widespread in foods and beverages which poses a serious threat to human health. However, no enzyme was known to be able to degrade this mycotoxin. For the first time, we discovered that a manganese peroxidase (MrMnP) from Moniliophthora roreri can efficiently degrade patulin. The MrMnP gene was cloned into pPICZα(A) and then the recombinant plasmid was transformed into Pichia pastoris X-33. The recombinant strain produced extracellular manganese peroxidase with an activity of up to 3659.5 U/L. The manganese peroxidase MrMnP was able to rapidly degrade patulin, with hydroascladiol appearing as a main degradation product. Five mg/L of pure patulin were completely degraded within 5 h. Moreover, up to 95% of the toxin was eliminated in a simulated patulin-contaminated apple juice after 24 h. Using Escherichia coli as a model, it was demonstrated that the deconstruction of patulin led to detoxification. Collectively, these traits make MrMnP an intriguing candidate useful in enzymatic detoxification of patulin in foods and beverages.
Keywords: apple juice; detoxification; manganese peroxidase; mycotoxin; patulin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Detoxification of the Mycotoxin Citrinin by a Manganese Peroxidase from Moniliophthora roreri.Toxins (Basel). 2022 Nov 18;14(11):801. doi: 10.3390/toxins14110801. Toxins (Basel). 2022. PMID: 36422974 Free PMC article.
-
Characterization of a short-chain dehydrogenase/reductase and its function in patulin biodegradation in apple juice.Food Chem. 2021 Jun 30;348:129046. doi: 10.1016/j.foodchem.2021.129046. Epub 2021 Jan 11. Food Chem. 2021. PMID: 33508606
-
Potential of two Metschnikowia pulcherrima (yeast) strains for in vitro biodegradation of patulin.J Food Prot. 2011 Jan;74(1):154-6. doi: 10.4315/0362-028X.JFP-10-331. J Food Prot. 2011. PMID: 21219780
-
Patulin in food: A mycotoxin concern for human health and its management strategies.Toxicon. 2021 Jul 30;198:12-23. doi: 10.1016/j.toxicon.2021.04.027. Epub 2021 Apr 29. Toxicon. 2021. PMID: 33933519 Review.
-
Progress in the distribution, toxicity, control, and detoxification of patulin: A review.Toxicon. 2020 Sep;184:83-93. doi: 10.1016/j.toxicon.2020.05.006. Epub 2020 Jun 2. Toxicon. 2020. PMID: 32502554 Review.
Cited by
-
Bioenzymatic detoxification of mycotoxins.Front Microbiol. 2024 Jul 18;15:1434987. doi: 10.3389/fmicb.2024.1434987. eCollection 2024. Front Microbiol. 2024. PMID: 39091297 Free PMC article. Review.
-
Bioprotective yeasts: Potential to limit postharvest spoilage and to extend shelf life or improve microbial safety of processed foods.Heliyon. 2024 Jan 21;10(3):e24929. doi: 10.1016/j.heliyon.2024.e24929. eCollection 2024 Feb 15. Heliyon. 2024. PMID: 38318029 Free PMC article. Review.
-
Exploring the cellulolytic and hemicellulolytic activities of manganese peroxidase for lignocellulose deconstruction.Biotechnol Biofuels Bioprod. 2023 Sep 19;16(1):139. doi: 10.1186/s13068-023-02386-0. Biotechnol Biofuels Bioprod. 2023. PMID: 37726830 Free PMC article.
-
Novel Strategies for the Biodegradation and Detoxification of Mycotoxins in Post-Harvest Grain.Toxins (Basel). 2023 Jul 5;15(7):445. doi: 10.3390/toxins15070445. Toxins (Basel). 2023. PMID: 37505714 Free PMC article.
-
Bacillus subtilis spore surface display enhances manganese peroxidase stability and stress resistance.Bioresour Bioprocess. 2025 Jun 10;12(1):57. doi: 10.1186/s40643-025-00901-9. Bioresour Bioprocess. 2025. PMID: 40490641 Free PMC article.
References
-
- Iqbal S.Z., Malik S., Asi M.R., Selamat J., Malik N. Natural occurrence of patulin in different fruits, juices and smoothies and evaluation of dietary intake in Punjab, Pakistan. Food Control. 2018;84:370–374. doi: 10.1016/j.foodcont.2017.08.024. - DOI
-
- Li N., Cui R., Zhang F., Meng X., Liu B. A novel enzyme from Rhodotorula mucilaginosa Aldolase: Isolation, identification and degradation for patulin in apple juice. Process Biochem. 2022;116:148–156. doi: 10.1016/j.procbio.2022.03.001. - DOI
-
- Yang Q., Ma J., Solairaj D., Fu Y., Zhang H. Efficacy of Meyerozyma guilliermondii in controlling patulin production by Penicillium expansum in shuijing pears. Biol. Control. 2022;168:104856. doi: 10.1016/j.biocontrol.2022.104856. - DOI
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources

