Exploring the Utility of Recombinant Snake Venom Serine Protease Toxins as Immunogens for Generating Experimental Snakebite Antivenoms
- PMID: 35878181
- PMCID: PMC9319908
- DOI: 10.3390/toxins14070443
Exploring the Utility of Recombinant Snake Venom Serine Protease Toxins as Immunogens for Generating Experimental Snakebite Antivenoms
Abstract
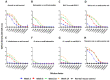
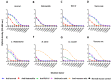
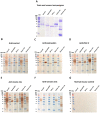
Snakebite is a neglected tropical disease that causes high rates of global mortality and morbidity. Although snakebite can cause a variety of pathologies in victims, haemotoxic effects are particularly common and are typically characterised by haemorrhage and/or venom-induced consumption coagulopathy. Despite polyclonal antibody-based antivenoms being the mainstay life-saving therapy for snakebite, they are associated with limited cross-snake species efficacy, as there is often extensive toxin variation between snake venoms, including those used as immunogens for antivenom production. This restricts the therapeutic utility of any antivenom to certain geographical regions. In this study, we explored the feasibility of using recombinantly expressed toxins as immunogens to stimulate focused, pathology-specific, antibodies in order to broadly counteract specific toxins associated with snakebite envenoming. Three snake venom serine proteases (SVSP) toxins, sourced from geographically diverse and medically important viper snake venoms, were successfully expressed in HEK293F mammalian cells and used for murine immunisation. Analyses of the resulting antibody responses revealed that ancrod and RVV-V stimulated the strongest immune responses, and that experimental antivenoms directed against these recombinant SVSP toxins, and a mixture of the three different immunogens, extensively recognised and exhibited immunological binding towards a variety of native snake venoms. While the experimental antivenoms showed some reduction in abnormal clotting parameters stimulated by the toxin immunogens and crude venom, specifically reducing the depletion of fibrinogen levels and prolongation of prothrombin times, fibrinogen degradation experiments revealed that they broadly protected against venom- and toxin-induced fibrinogenolytic functional activities. Overall, our findings further strengthen the case for the use of recombinant venom toxins as supplemental immunogens to stimulate focused and desirable antibody responses capable of neutralising venom-induced pathological effects, and therefore potentially circumventing some of the limitations associated with current snakebite therapies.
Keywords: antivenom; immunogen; neglected tropical diseases; polyclonal antibodies; recombinant expression; serine proteases; snake venom toxin; snakebite.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Commercial Antivenoms Exert Broad Paraspecific Immunological Binding and In Vitro Inhibition of Medically Important Bothrops Pit Viper Venoms.Toxins (Basel). 2022 Dec 20;15(1):1. doi: 10.3390/toxins15010001. Toxins (Basel). 2022. PMID: 36668821 Free PMC article.
-
Exploring the Utility of ssDNA Aptamers Directed against Snake Venom Toxins as New Therapeutics for Snakebite Envenoming.Toxins (Basel). 2022 Jul 8;14(7):469. doi: 10.3390/toxins14070469. Toxins (Basel). 2022. PMID: 35878207 Free PMC article.
-
Pathology-specific experimental antivenoms for haemotoxic snakebite: The impact of immunogen diversity on the in vitro cross-reactivity and in vivo neutralisation of geographically diverse snake venoms.PLoS Negl Trop Dis. 2021 Aug 18;15(8):e0009659. doi: 10.1371/journal.pntd.0009659. eCollection 2021 Aug. PLoS Negl Trop Dis. 2021. PMID: 34407084 Free PMC article.
-
A Review of the Proteomic Profiling of African Viperidae and Elapidae Snake Venoms and Their Antivenom Neutralisation.Toxins (Basel). 2022 Oct 22;14(11):723. doi: 10.3390/toxins14110723. Toxins (Basel). 2022. PMID: 36355973 Free PMC article. Review.
-
Deciphering toxico-proteomics of Asiatic medically significant venomous snake species: A systematic review and interactive data dashboard.Toxicon. 2024 Nov 6;250:108120. doi: 10.1016/j.toxicon.2024.108120. Epub 2024 Oct 10. Toxicon. 2024. PMID: 39393539
Cited by
-
Commercial Antivenoms Exert Broad Paraspecific Immunological Binding and In Vitro Inhibition of Medically Important Bothrops Pit Viper Venoms.Toxins (Basel). 2022 Dec 20;15(1):1. doi: 10.3390/toxins15010001. Toxins (Basel). 2022. PMID: 36668821 Free PMC article.
-
Exploring the Utility of ssDNA Aptamers Directed against Snake Venom Toxins as New Therapeutics for Snakebite Envenoming.Toxins (Basel). 2022 Jul 8;14(7):469. doi: 10.3390/toxins14070469. Toxins (Basel). 2022. PMID: 35878207 Free PMC article.
-
Exploring the Diversity and Function of Serine Proteases in Toxicofera Reptile Venoms: A Comprehensive Overview.Toxins (Basel). 2024 Oct 3;16(10):428. doi: 10.3390/toxins16100428. Toxins (Basel). 2024. PMID: 39453204 Free PMC article. Review.
-
Maggot Kinase and Natural Thrombolytic Proteins.ACS Omega. 2024 May 2;9(20):21768-21779. doi: 10.1021/acsomega.4c01663. eCollection 2024 May 21. ACS Omega. 2024. PMID: 38799322 Free PMC article. Review.
-
In vitro immunoreactivity and in vivo neutralization of Trimeresurus gracilis venom with antivenoms targeting four pit viper species.PLoS Negl Trop Dis. 2024 Mar 25;18(3):e0012070. doi: 10.1371/journal.pntd.0012070. eCollection 2024 Mar. PLoS Negl Trop Dis. 2024. PMID: 38527073 Free PMC article.
References
-
- Kasturiratne A., Wickremasinghe A.R., de Silva N., Gunawardena N.K., Pathmeswaran A., Premaratna R., Savioli L., Lalloo D.G., de Silva H.J. The global burden of snakebite: A literature analysis and modelling based on regional estimates of envenoming and deaths. PLoS Med. 2008;5:e218. doi: 10.1371/journal.pmed.0050218. - DOI - PMC - PubMed
-
- Williams D.J., Faiz M.A., Abela-Ridder B., Ainsworth S., Bulfone T.C., Nickerson A.D., Habib A.G., Junghanss T., Fan H.W., Turner M., et al. Strategy for a globally coordinated response to a priority neglected tropical disease: Snakebite envenoming. PLoS Negl. Trop. Dis. 2019;13:e0007059. doi: 10.1371/journal.pntd.0007059. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

