Evaluation of Acute and Subacute Toxicity and LC-MS/MS Compositional Alkaloid Determination of the Hydroethanolic Extract of Dysphania ambrosioides (L.) Mosyakin and Clemants Flowers
- PMID: 35878213
- PMCID: PMC9316831
- DOI: 10.3390/toxins14070475
Evaluation of Acute and Subacute Toxicity and LC-MS/MS Compositional Alkaloid Determination of the Hydroethanolic Extract of Dysphania ambrosioides (L.) Mosyakin and Clemants Flowers
Abstract
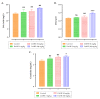
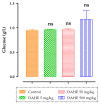
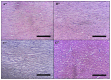
Dysphania ambrosioides (L.) Mosyakin and Clemants is a medicinal plant that has traditionally been used to cure a range of diseases. There has been no thorough investigation of the potential toxicity of this plant. The objective of this study is to assess the acute and subacute toxicity of D. ambrosioides hydroethanolic extract (DAHE), as well as it alkaloids composition, utilizing LC-MS/MS analysis. An in silico approach was applied to determine pharmacokinetic parameters and to predict the toxicity of D. ambrosioides identified alkaloids. A 14-day treatment with a single oral dose of 1-7 g/kg was carried out to investigate acute toxicity. DAHE was given orally at dosages of 5, 50, and 500 mg/kg for 15 days in the subacute toxicity investigation, and body weight and biochemical parameters were evaluated. Livers, kidneys, lungs, and heart were examined histologically. Chromatographic investigation revealed the existence of nine alkaloids, with N-formylnorgalanthamine being the most prevalent. The oral LD50 value of DAHE was found to be 5000 mg/kg in an acute toxicity study. No variations were observed with respect to food intake, water consumption, mortality, or body and organ weight in the subacute toxicity study. On the other hand, DAHE (500 mg/kg) significantly enhanced alanineaminotransferase, aspartate aminotransferase, and urea. Liver and kidney histological examinations revealed modest infiltration of hepatocyte trabeculae by inflammatory cells in the liver and slight alteration in the kidney histoarchitecture. According to our findings, DAHE exhibits low to moderate toxicity.
Keywords: ADMET analysis; Dysphania ambrosioides; LC-MS/MS; Mexican tea; acute toxicity; alkaloids; in silico; subacute toxicity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures









Similar articles
-
Phytochemical Analysis, Antispasmodic, Myorelaxant, and Antioxidant Effect of Dysphania ambrosioides (L.) Mosyakin and Clemants Flower Hydroethanolic Extracts and Its Chloroform and Ethyl Acetate Fractions.Molecules. 2021 Dec 1;26(23):7300. doi: 10.3390/molecules26237300. Molecules. 2021. PMID: 34885883 Free PMC article.
-
LC-MS/MS profiling and toxicological evaluation of Argania spinosa extract: Acute and subacute studies in Swiss Albino mice with in vivo and in silico approaches.J Ethnopharmacol. 2025 Jun 26;350:120009. doi: 10.1016/j.jep.2025.120009. Epub 2025 May 22. J Ethnopharmacol. 2025. PMID: 40412776
-
Possible Mechanism of Dysphania ambrosioides (L.) Mosyakin & Clemants Seed Extract Suppresses the Migration and Invasion of Human Hepatocellular Carcinoma Cells SMMC-7721.Chem Biodivers. 2023 Mar;20(3):e202200768. doi: 10.1002/cbdv.202200768. Epub 2023 Feb 15. Chem Biodivers. 2023. PMID: 36694378
-
Dysphania ambrosioides (L.) Mosyakin and Clemants: bridging traditional knowledge, photochemistry, preclinical investigations, and toxicological validation for health benefits.Naunyn Schmiedebergs Arch Pharmacol. 2024 Feb;397(2):969-1001. doi: 10.1007/s00210-023-02658-4. Epub 2023 Aug 8. Naunyn Schmiedebergs Arch Pharmacol. 2024. PMID: 37552317 Review.
-
Computational Evaluation of Bioactive Compounds from Dysphania ambrosioides Leaves.Chem Biodivers. 2024 Mar;21(3):e202301527. doi: 10.1002/cbdv.202301527. Epub 2024 Feb 29. Chem Biodivers. 2024. PMID: 38253787 Review.
Cited by
-
Chemico-pharmacological and computational studies of Ophiorrhiza fasciculata D. Don and Psychotria silhetensis Hook. f. focusing cytotoxic, thrombolytic, anti-inflammatory, antioxidant, and antibacterial properties.Heliyon. 2023 Sep 13;9(9):e20100. doi: 10.1016/j.heliyon.2023.e20100. eCollection 2023 Sep. Heliyon. 2023. PMID: 37809757 Free PMC article.
-
Screening of Phytochemical, Antimicrobial, and Antioxidant Properties of Juncus acutus from Northeastern Morocco.Life (Basel). 2023 Oct 29;13(11):2135. doi: 10.3390/life13112135. Life (Basel). 2023. PMID: 38004275 Free PMC article.
-
Evaluation of Antioxidant Activity, Cytotoxicity, and Genotoxicity of Ptychotis verticillata Essential Oil: Towards Novel Breast Cancer Therapeutics.Life (Basel). 2023 Jul 19;13(7):1586. doi: 10.3390/life13071586. Life (Basel). 2023. PMID: 37511960 Free PMC article.
-
Cataleptogenic Effect of Haloperidol Formulated in Water-Soluble Calixarene-Based Nanoparticles.Pharmaceutics. 2023 Mar 11;15(3):921. doi: 10.3390/pharmaceutics15030921. Pharmaceutics. 2023. PMID: 36986782 Free PMC article.
-
Pharmacological Properties of Chemically Characterized Extracts from Mastic Tree: In Vitro and In Silico Assays.Life (Basel). 2023 Jun 14;13(6):1393. doi: 10.3390/life13061393. Life (Basel). 2023. PMID: 37374175 Free PMC article.
References
-
- Christaki E., Bonos E., Giannenas I., Florou-Paneri P. Aromatic plants as a source of bioactive compounds. Agriculture. 2012;2:228–243. doi: 10.3390/agriculture2030228. - DOI
-
- Belakhdar J. La Pharmacopée Marocaine Traditionnelle. Medecine Arabe Ancienne et Savoirs Populaires. LE FENNEC EDIT; Casablanca, Morocco: 1997.
-
- Jouad H., Haloui M., Rhiouani H., ElHilaly J., Eddouks M. Ethnobotanical survey of medicinal plants used for the treatment of diabetes, cardiac and renal diseases in the North centre region of Morocco (Fez—Boulemane) J. Ethnopharmacol. 2001;77:175–182. doi: 10.1016/S0378-8741(01)00289-6. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

