Beneficial and Harmful Effects of Monoclonal Antibodies for the Treatment and Prophylaxis of COVID-19: Systematic Review and Meta-Analysis
- PMID: 35878688
- PMCID: PMC9307485
- DOI: 10.1016/j.amjmed.2022.06.019
Beneficial and Harmful Effects of Monoclonal Antibodies for the Treatment and Prophylaxis of COVID-19: Systematic Review and Meta-Analysis
Abstract
Background: We systematically assessed beneficial and harmful effects of monoclonal antibodies for coronavirus disease 2019 (COVID-19) treatment, and prophylaxis in individuals exposed to severe acute respiratory syndrome coronavirus 2.
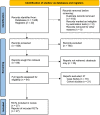
Methods: We searched 5 engines and 3 registries until November 3, 2021 for randomized controlled trials evaluating monoclonal antibodies vs control in hospitalized or non-hospitalized adults with COVID-19, or as prophylaxis. Primary outcomes were all-cause mortality, COVID-19-related death, and serious adverse events; hospitalization for non-hospitalized; and development of symptomatic COVID-19 for prophylaxis. Inverse variance random effects models were used for meta-analyses. Grading of Recommendations, Assessment, Development, and Evaluations methodology was used to assess certainty of evidence.
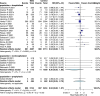
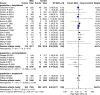
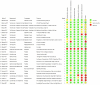
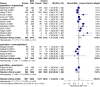
Results: Twenty-seven randomized controlled trials were included: 20 in hospitalized patients (n = 8253), 5 in non-hospitalized patients (n = 2922), and 2 in prophylaxis (n = 2680). In hospitalized patients, monoclonal antibodies slightly reduced mechanical ventilation (relative risk [RR] 0.74; 95% confidence interval [CI], 0.60-0.9; I2 = 20%, low certainty of evidence) and bacteremia (RR 0.77; 95% CI, 0.64-0.92; I2 = 7%, low certainty of evidence); evidence was very uncertain about the effect on adverse events (RR 1.31; 95% CI, 1.02-1.67; I2 = 77%, very low certainty of evidence). In non-hospitalized patients, monoclonal antibodies reduced hospitalizations (RR 0.30; 95% CI, 0.17-0.53; I2 = 0%, high certainty of evidence) and may slightly reduce serious adverse events (RR 0.47; 95% CI, 0.22-1.01; I2 = 33%, low certainty of evidence). In prophylaxis studies, monoclonal antibodies probably reduced viral load slightly (mean difference -0.8 log10; 95% CI, -1.21 to -0.39, moderate certainty of evidence). There were no effects on other outcomes.
Conclusions: Monoclonal antibodies had limited effects on most of the outcomes in COVID-19 patients, and when used as prophylaxis. Additional data are needed to determine their efficacy and safety.
Keywords: COVID-19; Meta-analysis; Monoclonal antibodies; Prophylaxis; Treatment.
Copyright © 2022 Elsevier Inc. All rights reserved.
Figures





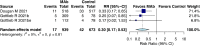
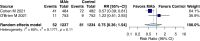
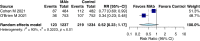




















References
-
- Worldometers. COVID-19 statistics. Available at: https://www.worldometers.info/coronavirus/. Accessed March 28, 2022.
-
- Infectious Diseases Society of America. Anti-SARS-CoV-2 monoclonal antibodies. Available at:https://www.idsociety.org/covid-19-real-time-learning-network/therapeuti.... Accessed March 28, 2022.
-
- Food and Drug Administration. Emergency use authorization of drugs and non-vaccine biological products. Available at:https://www.fda.gov/emergency-preparedness-and-response/mcm-legal-regula.... Accessed March 28, 2022.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

