Mice lacking proSAAS display alterations in emotion, consummatory behavior and circadian entrainment
- PMID: 35878875
- PMCID: PMC9444949
- DOI: 10.1111/gbb.12827
Mice lacking proSAAS display alterations in emotion, consummatory behavior and circadian entrainment
Abstract
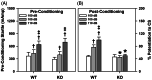
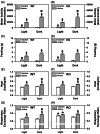
ProSAAS is a neuroendocrine protein that is cleaved by neuropeptide-processing enzymes into more than a dozen products including the bigLEN and PEN peptides, which bind and activate the receptors GPR171 and GPR83, respectively. Previous studies have suggested that proSAAS-derived peptides are involved in physiological functions that include body weight regulation, circadian rhythms and anxiety-like behavior. In the present study, we find that proSAAS knockout mice display robust anxiety-like behaviors in the open field, light-dark emergence and elevated zero maze tests. These mutant mice also show a reduction in cued fear and an impairment in fear-potentiated startle, indicating an important role for proSAAS-derived peptides in emotional behaviors. ProSAAS knockout mice exhibit reduced water consumption and urine production relative to wild-type controls. No differences in food consumption and overall energy expenditure were observed between the genotypes. However, the respiratory exchange ratio was elevated in the mutants during the light portion of the light-dark cycle, indicating decreased fat metabolism during this period. While proSAAS knockout mice show normal circadian patterns of activity, even upon long-term exposure to constant darkness, they were unable to shift their circadian clock upon exposure to a light pulse. Taken together, these results show that proSAAS-derived peptides modulate a wide range of behaviors including emotion, metabolism and the regulation of the circadian clock.
Keywords: anxiety; circadian activity; fear; metabolism; mice; proSAAS knockout.
© 2022 The Authors. Genes, Brain and Behavior published by International Behavioural and Neural Genetics Society and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no potential conflict of interest.
Figures




Similar articles
-
GPR171 is a hypothalamic G protein-coupled receptor for BigLEN, a neuropeptide involved in feeding.Proc Natl Acad Sci U S A. 2013 Oct 1;110(40):16211-6. doi: 10.1073/pnas.1312938110. Epub 2013 Sep 16. Proc Natl Acad Sci U S A. 2013. PMID: 24043826 Free PMC article.
-
ProSAAS neuropeptides and receptors GPR171 and GPR83: Potential therapeutic applications for pain, anxiety, and body weight regulation.J Pharmacol Exp Ther. 2025 Jun;392(6):103599. doi: 10.1016/j.jpet.2025.103599. Epub 2025 May 5. J Pharmacol Exp Ther. 2025. PMID: 40450835 Review.
-
ProSAAS-derived peptides are colocalized with neuropeptide Y and function as neuropeptides in the regulation of food intake.PLoS One. 2011;6(12):e28152. doi: 10.1371/journal.pone.0028152. Epub 2011 Dec 2. PLoS One. 2011. PMID: 22164236 Free PMC article.
-
The propeptide precursor proSAAS is involved in fetal neuropeptide processing and body weight regulation.J Neurochem. 2010 Jun;113(5):1275-84. doi: 10.1111/j.1471-4159.2010.06706.x. Epub 2010 Mar 26. J Neurochem. 2010. PMID: 20367757 Free PMC article.
-
An essential role for peptidergic signalling in the control of circadian rhythms in the suprachiasmatic nuclei.J Neuroendocrinol. 2003 Apr;15(4):335-8. doi: 10.1046/j.1365-2826.2003.01005.x. J Neuroendocrinol. 2003. PMID: 12622830 Review.
Cited by
-
The neuronal chaperone proSAAS is highly expressed in the retina.PLoS One. 2025 May 16;20(5):e0321867. doi: 10.1371/journal.pone.0321867. eCollection 2025. PLoS One. 2025. PMID: 40378115 Free PMC article.
-
A small molecule ligand for the novel pain target, GPR171, produces minimal reward in mice.Pharmacol Biochem Behav. 2023 Mar;224:173543. doi: 10.1016/j.pbb.2023.173543. Epub 2023 Mar 17. Pharmacol Biochem Behav. 2023. PMID: 36933620 Free PMC article.
-
Spatial transcriptomics reveal neuron-astrocyte synergy in long-term memory.Nature. 2024 Mar;627(8003):374-381. doi: 10.1038/s41586-023-07011-6. Epub 2024 Feb 7. Nature. 2024. PMID: 38326616 Free PMC article.
References
-
- Fricker LD. Neuropeptides and other bioactive peptides: from discovery to function. Colloquium Series on Neuropeptides. Morgan & Claypool Life Sciences; 2012:122‐133.
-
- Fricker LD. Carboxypeptidase E. Annu Rev Physiol. 1988;50:309‐321. - PubMed
-
- Cameron A, Fortenberry Y, Lindberg I. The SAAS granin exhibits structural and functional homology to 7B2 and contains a highly potent hexapeptide inhibitor of PC1. FEBS Lett. 2000;473:135‐138. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

