Safety of breast feeding during rituximab treatment in multiple sclerosis
- PMID: 35879056
- PMCID: PMC9763193
- DOI: 10.1136/jnnp-2022-329545
Safety of breast feeding during rituximab treatment in multiple sclerosis
Abstract
Background: There are limited data on the safety of breast feeding during rituximab therapy. Our objective is to determine exposure from breast feeding and biological effects of rituximab in breastfed infants.
Methods: In our case series of six mother-infant pairs, the nursing mothers with relapsing-remitting multiple sclerosis received rituximab during breast feeding. As part of clinical follow-up, six serial breast milk samples, and blood samples from both mothers and infants, were collected and analysed.
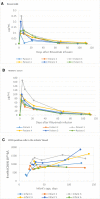
Results: The median average rituximab concentration (Cavg) in breast milk was 0.04 µg/mL and the estimated relative infant dose (RID) was 0.07%. The highest measured concentration of rituximab in the breast milk samples was 0.25 µg/mL, giving an estimated RID of 0.26%.All infant serum rituximab concentrations were below 0.01 µg/mL. The CD19 +B cell count values were within the 10th- 90th percentiles of reported normal ranges in healthy infants.
Conclusions: We found minimal transfer of rituximab into breast milk and could not reliably detect levels of rituximab in infant serum. B cell counts in infants were unaffected.
Keywords: multiple sclerosis.
© Author(s) (or their employer(s)) 2022. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: BER reports no disclosures. ØT received speaker honoraria from and served on scientific advisory boards for Biogen, Sanofi-Aventis, Merck and Novartis. K-MM has received scientific advisory board or speaker honoraria from Biogen, Novartis and Roche, and has participated in clinical trials organised by Biogen, Merck, Novartis, Roche and Sanofi. LB has received unrestricted research grants to his institution and speaker honoraria from Almirall, Biogen, Genzyme, Merck, Novartis, Roche, and Teva, and has participated in clinical trials organised by Biogen, Merck, Novartis, Roche and Genzyme. SW has received honoraria from Biogen, Novartis and Sanofi.
Figures
Similar articles
-
Minimal breast milk transfer of rituximab, a monoclonal antibody used in neurological conditions.Neurol Neuroimmunol Neuroinflamm. 2019 Nov 12;7(1):e637. doi: 10.1212/NXI.0000000000000637. Print 2020 Jan. Neurol Neuroimmunol Neuroinflamm. 2019. PMID: 31719115 Free PMC article.
-
Exposure Concentrations of Infants Breastfed by Women Receiving Biologic Therapies for Inflammatory Bowel Diseases and Effects of Breastfeeding on Infections and Development.Gastroenterology. 2018 Sep;155(3):696-704. doi: 10.1053/j.gastro.2018.05.040. Epub 2018 May 30. Gastroenterology. 2018. PMID: 29857090
-
Is there any difference between the iodine statuses of breast-fed and formula-fed infants and their mothers in an area with iodine sufficiency?Br J Nutr. 2018 May;119(9):1012-1018. doi: 10.1017/S0007114518000351. Epub 2018 Mar 5. Br J Nutr. 2018. PMID: 29502541
-
Oral galactagogues (natural therapies or drugs) for increasing breast milk production in mothers of non-hospitalised term infants.Cochrane Database Syst Rev. 2020 May 18;5(5):CD011505. doi: 10.1002/14651858.CD011505.pub2. Cochrane Database Syst Rev. 2020. PMID: 32421208 Free PMC article.
-
Infant drug exposure via breast milk.Br J Clin Pharmacol. 2022 Oct;88(10):4311-4327. doi: 10.1111/bcp.14538. Epub 2020 Sep 13. Br J Clin Pharmacol. 2022. PMID: 32860456 Review.
Cited by
-
[Esclerosis multiple. Lactancia. Lactante. Planificacion familiar. Posparto. Tratamiento modificador de la enfermedad.].Rev Neurol. 2023 Jan 1;76(1):21-30. doi: 10.33588/rn.7601.2022404. Rev Neurol. 2023. PMID: 36544373 Free PMC article. Review. Spanish.
-
Topical review: Lactation and use of DMTs in women with MS.Mult Scler. 2024 Nov;30(13):1578-1591. doi: 10.1177/13524585241257843. Epub 2024 Sep 30. Mult Scler. 2024. PMID: 39348090 Free PMC article. Review.
-
Patient-centered pregnancy planning in multiple sclerosis: evidence for a new era.Arq Neuropsiquiatr. 2024 Oct;82(10):1-11. doi: 10.1055/s-0044-1791202. Epub 2024 Oct 2. Arq Neuropsiquiatr. 2024. PMID: 39357853 Free PMC article. Review.
-
Safety of Drugs in Breastfeeding Women With CKD.Kidney Int Rep. 2025 Apr 27;10(7):2189-2201. doi: 10.1016/j.ekir.2025.04.038. eCollection 2025 Jul. Kidney Int Rep. 2025. PMID: 40677356 Free PMC article.
-
A multi-country cohort database study to assess pregnancy and infant outcomes after potential maternal or paternal exposure to cladribine tablets in the treatment of multiple sclerosis: the CLEAR study methods and status update.Ther Adv Neurol Disord. 2025 Jan 27;18:17562864241310996. doi: 10.1177/17562864241310996. eCollection 2025. Ther Adv Neurol Disord. 2025. PMID: 39872126 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Research Materials
