The first Japanese biobank of patient-derived pediatric acute lymphoblastic leukemia xenograft models
- PMID: 35879192
- PMCID: PMC9633318
- DOI: 10.1111/cas.15506
The first Japanese biobank of patient-derived pediatric acute lymphoblastic leukemia xenograft models
Abstract
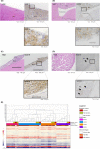
A lack of practical resources in Japan has limited preclinical discovery and testing of therapies for pediatric relapsed and refractory acute lymphoblastic leukemia (ALL), which has poor outcomes. Here, we established 57 patient-derived xenografts (PDXs) in NOD.Cg-Prkdcscid ll2rgtm1Sug /ShiJic (NOG) mice and created a biobank by preserving PDX cells including three extramedullary relapsed ALL PDXs. We demonstrated that our PDX mice and PDX cells mimicked the biological features of relapsed ALL and that PDX models reproduced treatment-mediated clonal selection. Our PDX biobank is a useful scientific resource for capturing drug sensitivity features of pediatric patients with ALL, providing an essential tool for the development of targeted therapies.
Keywords: biobank; leukemia; pediatric; preclinical; xenograft.
© 2022 The Authors. Cancer Science published by John Wiley & Sons Australia, Ltd on behalf of Japanese Cancer Association.
Figures




References
-
- Pui CH, Sandlund JT, Pei D, et al. Improved outcome for children with acute lymphoblastic leukemia: results of Total therapy study XIIIB at St Jude Children's research hospital. Blood. 2004;104(9):2690‐2696. - PubMed
-
- Gao H, Korn JM, Ferretti S, et al. High‐throughput screening using patient‐derived tumor xenografts to predict clinical trial drug response. Nat Med. 2015;21(11):1318‐1325. - PubMed
-
- Izumchenko E, Meir J, Bedi A, Wysocki PT, Hoque MO, Sidransky D. Patient‐derived xenografts as tools in pharmaceutical development. Clin Pharmacol Ther. 2016;99(6):612‐621. - PubMed
-
- Jones L, Carol H, Evans K, et al. A review of new agents evaluated against pediatric acute lymphoblastic leukemia by the pediatric preclinical testing program. Leukemia. 2016;30(11):2133‐2141. - PubMed

