A secretory form of Parkin-independent mitophagy contributes to the repertoire of extracellular vesicles released into the tumour interstitial fluid in vivo
- PMID: 35879267
- PMCID: PMC9314315
- DOI: 10.1002/jev2.12244
A secretory form of Parkin-independent mitophagy contributes to the repertoire of extracellular vesicles released into the tumour interstitial fluid in vivo
Abstract
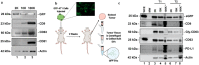
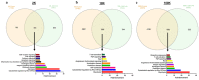
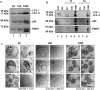
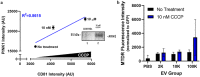
We characterized the in vivo interstitial fluid (IF) content of extracellular vesicles (EVs) using the GFP-4T1 syngeneic murine cancer model to study EVs in-transit to the draining lymph node. GFP labelling confirmed the IF EV tumour cell origin. Molecular analysis revealed an abundance of IF EV-associated proteins specifically involved in mitophagy and secretory autophagy. A set of proteins required for sequential steps of fission-induced mitophagy preferentially populated the CD81+/PD-L1+ IF EVs; PINK1, TOM20, and ARIH1 E3 ubiquitin ligase (required for Parkin-independent mitophagy), DRP1 and FIS1 (mitochondrial peripheral fission), VDAC-1 (ubiquitination state triggers mitophagy away from apoptosis), VPS35, SEC22b, and Rab33b (vacuolar sorting). Comparing in vivo IF EVs to in vitro EVs revealed 40% concordance, with an elevation of mitophagy proteins in the CD81+ EVs for both murine and human cell lines subjected to metabolic stress. The export of cellular mitochondria proteins to CD81+ EVs was confirmed by density gradient isolation from the bulk EV isolate followed by anti-CD81 immunoprecipitation, molecular sieve chromatography, and MitoTracker export into CD81+ EVs. We propose the 4T1 in vivo model as a versatile tool to functionally characterize IF EVs. IF EV export of fission mitophagy proteins has broad implications for mitochondrial function and cellular immunology.
Keywords: autophagosome; autophagy; breast cancer; extracellular vesicle; mitochondria; mitophagy.
© 2022 The Authors. Journal of Extracellular Vesicles published by Wiley Periodicals, LLC on behalf of the International Society for Extracellular Vesicles.
Conflict of interest statement
None.
Figures




References
-
- Baklaushev, V. P. , Kilpelã¤Inen, A. , Petkov, S. , Abakumov, M. A. , Grinenko, N. F. , Yusubalieva, G. M. , Latanova, A. A. , Gubskiy, I. L. , Zabozlaev, F. G. , Starodubova, E. S. , Abakumova, T. O. , Isaguliants, M. G. , & Chekhonin, V. P. (2017). Luciferase expression allows bioluminescence imaging but imposes limitations on the orthotopic mouse (4T1) model of breast cancer. Scientific Reports, 7, 7715. - PMC - PubMed
-
- Bosiljcic, M. , Hamilton, M. J. , Banath, J. P. , Lepard, N. E. , Mcdougal, D. C. , Jia, J. X. , Krystal, G. , & Bennewith, K. L. (2011). Myeloid suppressor cells regulate the lung environment—Letter: Figure 1. Cancer Research, 71, 5050–5051. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

