Extracellular vesicles engineered to bind albumin demonstrate extended circulation time and lymph node accumulation in mouse models
- PMID: 35879268
- PMCID: PMC9314316
- DOI: 10.1002/jev2.12248
Extracellular vesicles engineered to bind albumin demonstrate extended circulation time and lymph node accumulation in mouse models
Abstract
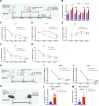
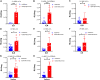
Extracellular vesicles (EVs) have shown promise as potential therapeutics for the treatment of various diseases. However, their rapid clearance after administration could be a limitation in certain therapeutic settings. To solve this, an engineering strategy is employed to decorate albumin onto the surface of the EVs through surface display of albumin binding domains (ABDs). ABDs were either included in the extracellular loops of select EV-enriched tetraspanins (CD63, CD9 and CD81) or directly fused to the extracellular terminal of single transmembrane EV-sorting domains, such as Lamp2B. These engineered EVs exert robust binding capacity to human serum albumins (HSA) in vitro and mouse serum albumins (MSA) after injection in mice. By binding to MSA, circulating time of EVs dramatically increases after different routes of injection in different strains of mice. Moreover, these engineered EVs show considerable lymph node (LN) and solid tumour accumulation, which can be utilized when using EVs for immunomodulation, cancer- and/or immunotherapy. The increased circulation time of EVs may also be important when combined with tissue-specific targeting ligands and could provide significant benefit for their therapeutic use in a variety of disease indications.
Keywords: albumin binding domains; circulation time; extracellular vesicles; lymph node accumulation; tetraspanins.
© 2022 The Authors. Journal of Extracellular Vesicles published by Wiley Periodicals, LLC on behalf of the International Society for Extracellular Vesicles.
Conflict of interest statement
Oscar P. B. Wiklander, Joel Z. Nordin, Dhanu Gupta, Samir E. L. Andaloussi are consultants and stakeholders in Evox Therapeutics Limited, Oxford, United Kingdom. Valentina Galli, Nathalie Howe, Christopher Davies, Justin Hean, Eleni Kyriakopoulou are employees of Evox Therapeutics Limited, Oxford, United Kingdom. Other authors declare no conflict of interest.
Figures






Similar articles
-
Framework for rapid comparison of extracellular vesicle isolation methods.Elife. 2021 Nov 16;10:e70725. doi: 10.7554/eLife.70725. Elife. 2021. PMID: 34783650 Free PMC article.
-
Tetraspanins distinguish separate extracellular vesicle subpopulations in human serum and plasma - Contributions of platelet extracellular vesicles in plasma samples.J Extracell Vesicles. 2022 May;11(5):e12213. doi: 10.1002/jev2.12213. J Extracell Vesicles. 2022. PMID: 35524458 Free PMC article.
-
Quantification of protein cargo loading into engineered extracellular vesicles at single-vesicle and single-molecule resolution.J Extracell Vesicles. 2021 Aug;10(10):e12130. doi: 10.1002/jev2.12130. Epub 2021 Aug 2. J Extracell Vesicles. 2021. PMID: 34377376 Free PMC article.
-
Methods for loading therapeutics into extracellular vesicles and generating extracellular vesicles mimetic-nanovesicles.Methods. 2020 May 1;177:103-113. doi: 10.1016/j.ymeth.2020.01.001. Epub 2020 Jan 7. Methods. 2020. PMID: 31917274 Review.
-
Achieving the Promise of Therapeutic Extracellular Vesicles: The Devil is in Details of Therapeutic Loading.Pharm Res. 2017 May;34(5):1053-1066. doi: 10.1007/s11095-017-2123-5. Epub 2017 Mar 17. Pharm Res. 2017. PMID: 28315083 Free PMC article. Review.
Cited by
-
Cell-specific targeting of extracellular vesicles through engineering the glycocalyx.J Extracell Vesicles. 2022 Dec;11(12):e12290. doi: 10.1002/jev2.12290. J Extracell Vesicles. 2022. PMID: 36463392 Free PMC article.
-
Novel Endogenous Engineering Platform for Robust Loading and Delivery of Functional mRNA by Extracellular Vesicles.Adv Sci (Weinh). 2024 Nov;11(42):e2407619. doi: 10.1002/advs.202407619. Epub 2024 Sep 9. Adv Sci (Weinh). 2024. PMID: 39246205 Free PMC article.
-
The Lymphatic Vascular System in Extracellular Vesicle-Mediated Tumor Progression.Cancers (Basel). 2024 Dec 2;16(23):4039. doi: 10.3390/cancers16234039. Cancers (Basel). 2024. PMID: 39682225 Free PMC article. Review.
-
Nanoparticle Targeting Strategies for Lipid and Polymer-Based Gene Delivery to Immune Cells In Vivo.Small Sci. 2024 Jul 30;4(9):2400248. doi: 10.1002/smsc.202400248. eCollection 2024 Sep. Small Sci. 2024. PMID: 40212067 Free PMC article.
-
Advances in Therapeutic Applications of Extracellular Vesicles.Int J Nanomedicine. 2023 Jun 16;18:3285-3307. doi: 10.2147/IJN.S409588. eCollection 2023. Int J Nanomedicine. 2023. PMID: 37346366 Free PMC article. Review.
References
-
- Andersen, J. T. , Cameron, J. , Plumridge, A. , Evans, L. , Sleep, D. , & Sandlie, I. (2013). Single‐chain variable fragment albumin fusions bind the neonatal Fc receptor (FcRn) in a species‐dependent manner: Implications for in vivo half‐life evaluation of albumin fusion therapeutics. Journal of Biological Chemistry, 288(33), 24277–24285. 10.1074/jbc.M113.463000 - DOI - PMC - PubMed
-
- Belhadj, Z. , He, B. , Deng, H. , Song, S. , Zhang, H. , Wang, X. , Dai, W. , & Zhang, Q. (2020). A combined “eat me/don't eat me” strategy based on extracellular vesicles for anticancer nanomedicine. Journal of Extracellular Vesicles, 9(1), 1806444. 10.1080/20013078.2020.1806444 - DOI - PMC - PubMed
-
- Buatois, V. , Johnson, Z. , Salgado‐Pires, S. , Papaioannou, A. , Hatterer, E. , Chauchet, X. , Richard, F. , Barba, L. , Daubeuf, B. , Cons, L. , Broyer, L. , D'Asaro, M. , Matthes, T. , LeGallou, S. , Fest, T. , Tarte, K. , Clarke Hinojosa, R. K. , Ferrer, E. G. , Ribera, J. M. , …, & Ferlin, W. G. (2018). Preclinical development of a bispecific antibody that safely and effectively targets CD19 and CD47 for the treatment of B‐cell lymphoma and leukemia. Molecular Cancer Therapeutics, 17(8), 1739–1751. 10.1158/1535-7163.MCT-17-1095 - DOI - PMC - PubMed
-
- Chao, M. P. , Jaiswal, S. , Weissman‐Tsukamoto, R. , Alizadeh, A. A. , Gentles, A. J. , Volkmer, J. , Weiskopf, K. , Willingham, S. B. , Raveh, T. , Park, C. Y. , Majeti, R. , & Weissman, I. L. (2010). Calreticulin is the dominant pro‐phagocytic signal on multiple human cancers and is counterbalanced by CD47. Science Translational Medicine, 2(63), 63ra94. 10.1126/scitranslmed.3001375 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

