Activation of the JNKs/ATM-p53 axis is indispensable for the cytoprotection of dermal fibroblasts exposed to UVB radiation
- PMID: 35879280
- PMCID: PMC9314411
- DOI: 10.1038/s41419-022-05106-y
Activation of the JNKs/ATM-p53 axis is indispensable for the cytoprotection of dermal fibroblasts exposed to UVB radiation
Abstract
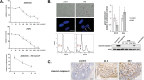
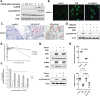
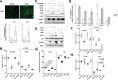
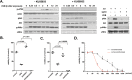
Although UVB radiation is mainly absorbed by the epidermis, ~5-10% of its photons reach and affect the upper part of the dermis. Physiologically relevant UVB doses, able to provoke erythema, induce apoptosis in human dermal fibroblasts in vitro, as well as in the dermis of SKH-1 mice. Given the sparse and even contradictory existing information on the effect of UVB radiation on dermal fibroblasts' viability, aim of this work was to unravel the crucial signaling pathways regulating the survival of UVB-treated human dermal fibroblasts. We found that UVB radiation immediately stimulates the phosphorylation of MAPK family members, as well as Akt, and is genotoxic leading to the delayed ATM-p53 axis activation. Akt phosphorylation after UVB radiation is EGFR-mediated and EGFR inhibition leads to a further decrease of viability, while the Akt activator SC79 rescues fibroblasts to an extent by a mechanism involving Nrf2 activation. The known Nrf2 activator sulforaphane also exerts a partial protective effect, although by acting in a distinct mechanism from SC79. On the other hand, inhibition of JNKs or of the ATM-p53 axis leads to a complete loss of viability after UVB irradiation. Interestingly, JNKs activation is necessary for p53 phosphorylation, while the ATM-p53 pathway is required for the long-term activation of JNKs and Akt, reassuring the protection from UVB. Although UVB radiation results in intense and prolonged increase of intracellular ROS levels, classical anti-oxidants, such as Trolox, are unable to affect Akt, JNKs, or p53 phosphorylation and to reverse the loss of fibroblasts' viability. Collectively, here we provide evidence that the main viability-regulating UVB-triggered biochemical pathways act synergistically towards the protection of human dermal fibroblasts, with EGFR/Akt and Nrf2 serving as auxiliary anti-apoptotic machineries, while JNKs/ATM-p53 activation and interplay being overriding and indispensable for the perpetuation of cellular defense and the maintenance of cell viability.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

