Molecular Cloning and Characterization of a Novel Exo-β-1,3-Galactanase from Penicillium oxalicum sp. 68
- PMID: 35879293
- PMCID: PMC9628948
- DOI: 10.4014/jmb.2204.04012
Molecular Cloning and Characterization of a Novel Exo-β-1,3-Galactanase from Penicillium oxalicum sp. 68
Abstract
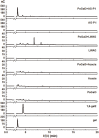
Arabinogalactans have diverse biological properties and can be used as pharmaceutical agents. Most arabinogalactans are composed of β-(1→3)-galactan, so it is particularly important to identify β-1,3-galactanases that can selectively degrade them. In this study, a novel exo-β-1,3-galactanase, named PoGal3, was screened from Penicillium oxalicum sp. 68, and hetero-expressed in P. pastoris GS115 as a soluble protein. PoGal3 belongs to glycoside hydrolase family 43 (GH43) and has a 1,356-bp gene length that encodes 451 amino acids residues. To study the enzymatic properties and substrate selectivity of PoGal3, β-1,3-galactan (AG-P-I) from larch wood arabinogalactan (LWAG) was prepared and characterized by HPLC and NMR. Using AG-P-I as substrate, purified PoGal3 exhibited an optimal pH of 5.0 and temperature of 40°C. We also discovered that Zn2+ had the strongest promoting effect on enzyme activity, increasing it by 28.6%. Substrate specificity suggests that PoGal3 functions as an exo-β-1,3-galactanase, with its greatest catalytic activity observed on AG-P-I. Hydrolytic products of AG-P-I are mainly composed of galactose and β-1,6-galactobiose. In addition, PoGal3 can catalyze hydrolysis of LWAG to produce galacto-oligomers. PoGal3 is the first enzyme identified as an exo-β-1,3-galactanase that can be used in building glycan blocks of crucial glycoconjugates to assess their biological functions.
Keywords: Exo-β-1,3-galactanase; Penicillium oxalicum; glycoside hydrolase family 43; larch wood arabinogalactans.
Conflict of interest statement
The authors have no financial conflicts of interest to declare.
Figures


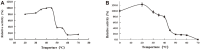
References
-
- Goellner EM, Utermoehlen J, Kramer R, Classen B. Structure of arabinogalactan from Larix laricina and its reactivity with antibodies directed against type-II-arabinogalactans. Carbohydr. Polym. 2011;86:1739–1744. doi: 10.1016/j.carbpol.2011.07.006. - DOI
-
- Prescott JH, Groman EV, Gulyas G. New molecular weight forms of arabinogalactan from Larix occidentalis. Carbohydr. Res. 1997;301:89–93. doi: 10.1016/S0008-6215(97)00078-5. - DOI

