Lymphatic-preserving treatment sequencing with immune checkpoint inhibition unleashes cDC1-dependent antitumor immunity in HNSCC
- PMID: 35879302
- PMCID: PMC9314425
- DOI: 10.1038/s41467-022-31941-w
Lymphatic-preserving treatment sequencing with immune checkpoint inhibition unleashes cDC1-dependent antitumor immunity in HNSCC
Abstract
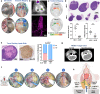
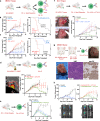
Despite the promise of immune checkpoint inhibition (ICI), therapeutic responses remain limited. This raises the possibility that standard of care treatments delivered in concert may compromise the tumor response. To address this, we employ tobacco-signature head and neck squamous cell carcinoma murine models in which we map tumor-draining lymphatics and develop models for regional lymphablation with surgery or radiation. We find that lymphablation eliminates the tumor ICI response, worsening overall survival and repolarizing the tumor- and peripheral-immune compartments. Mechanistically, within tumor-draining lymphatics, we observe an upregulation of conventional type I dendritic cells and type I interferon signaling and show that both are necessary for the ICI response and lost with lymphablation. Ultimately, we provide a mechanistic understanding of how standard oncologic therapies targeting regional lymphatics impact the tumor response to immune-oncology therapy in order to define rational, lymphatic-preserving treatment sequences that mobilize systemic antitumor immunity, achieve optimal tumor responses, control regional metastatic disease, and confer durable antitumor immunity.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA: A Cancer J. Clin. 2020;70:7–30. - PubMed
-
- Loganathan, S. K. et al. Rare driver mutations in head and neck squamous cell carcinomas converge on NOTCH signaling. https://www.science.org/doi/epdf/10.1126/science.aax0902 (2020). - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

