Machine learning-based analysis of overall stability constants of metal-ligand complexes
- PMID: 35879384
- PMCID: PMC9314427
- DOI: 10.1038/s41598-022-15300-9
Machine learning-based analysis of overall stability constants of metal-ligand complexes
Abstract
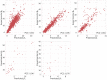
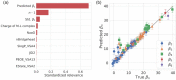
The stability constants of metal(M)-ligand(L) complexes are industrially important because they affect the quality of the plating film and the efficiency of metal separation. Thus, it is desirable to develop an effective screening method for promising ligands. Although there have been several machine-learning approaches for predicting stability constants, most of them focus only on the first overall stability constant of M-L complexes, and the variety of cations is also limited to less than 20. In this study, two Gaussian process regression models are developed to predict the first overall stability constant and the n-th (n > 1) overall stability constants. Furthermore, the feature relevance is quantitatively evaluated via sensitivity analysis. As a result, the electronegativities of both metal and ligand are found to be the most important factor for predicting the first overall stability constant. Interestingly, the predicted value of the first overall stability constant shows the highest correlation with the n-th overall stability constant of the corresponding M-L pair. Finally, the number of features is optimized using validation data where the ligands are not included in the training data, which indicates high generalizability. This study provides valuable insights and may help accelerate molecular screening and design for various applications.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
Applied machine learning for predicting the lanthanide-ligand binding affinities.Sci Rep. 2020 Aug 31;10(1):14322. doi: 10.1038/s41598-020-71255-9. Sci Rep. 2020. PMID: 32868845 Free PMC article.
-
Selectivity of the highly preorganized tetradentate ligand 2,9-di(pyrid-2-yl)-1,10-phenanthroline for metal ions in aqueous solution, including lanthanide(III) ions and the uranyl(VI) cation.Inorg Chem. 2013 Jan 7;52(1):15-27. doi: 10.1021/ic3002509. Epub 2012 Dec 11. Inorg Chem. 2013. PMID: 23231454
-
Protonation constant of monoaza-12-crown-4 ether and stability constants with selected metal ions in aqueous solution in the presence of an excess of sodium ion: a potentiometric and differential pulse polarographic study at fixed ligand to metal ratio and varied pH.Talanta. 1998 Dec;47(5):1175-89. doi: 10.1016/s0039-9140(98)00203-3. Talanta. 1998. PMID: 18967423
-
Progress towards machine learning reaction rate constants.Phys Chem Chem Phys. 2022 Feb 2;24(5):2692-2705. doi: 10.1039/d1cp04422b. Phys Chem Chem Phys. 2022. PMID: 34935798 Review.
-
Supervised Machine Learning Methods Applied to Predict Ligand- Binding Affinity.Curr Med Chem. 2017;24(23):2459-2470. doi: 10.2174/0929867324666170623092503. Curr Med Chem. 2017. PMID: 28641555 Review.
Cited by
-
Stability Constant and Potentiometric Sensitivity of Heavy Metal-Organic Fluorescent Compound Complexes: QSPR Models for Prediction and Design of Novel Coumarin-like Ligands.Toxics. 2023 Jul 7;11(7):595. doi: 10.3390/toxics11070595. Toxics. 2023. PMID: 37505560 Free PMC article.
References
-
- Kanani, N. Electroplating: Basic Principles, Processes and Practice 1st edition (Elsevier, 2004).
-
- Singh, J., Srivastava, A. N., Singh, N. & Singh, A. Stability Constants of Metal Complexes in Solution. in Stability and Applications of Coordination Compounds (ed. Srivastava, A. N.) (IntechOpen, 2019).
-
- Treybal, R. E. Mass transfer Operations (Springer, 1980).
-
- Dimmock PW, Warwick P, Robbins RA. Approaches to predicting stability constants. Analyst. 1995;120:2159–2170. doi: 10.1039/an9952002159. - DOI
LinkOut - more resources
Full Text Sources
Research Materials

