Glial cells as integrators of peripheral and central signals in the regulation of energy homeostasis
- PMID: 35879459
- PMCID: PMC7613794
- DOI: 10.1038/s42255-022-00610-z
Glial cells as integrators of peripheral and central signals in the regulation of energy homeostasis
Abstract
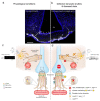
Communication between the periphery and the brain is key for maintaining energy homeostasis. To do so, peripheral signals from the circulation reach the brain via the circumventricular organs (CVOs), which are characterized by fenestrated vessels lacking the protective blood-brain barrier (BBB). Glial cells, by virtue of their plasticity and their ideal location at the interface of blood vessels and neurons, participate in the integration and transmission of peripheral information to neuronal networks in the brain for the neuroendocrine control of whole-body metabolism. Metabolic diseases, such as obesity and type 2 diabetes, can disrupt the brain-to-periphery communication mediated by glial cells, highlighting the relevance of these cell types in the pathophysiology of such complications. An improved understanding of how glial cells integrate and respond to metabolic and humoral signals has become a priority for the discovery of promising therapeutic strategies to treat metabolic disorders. This Review highlights the role of glial cells in the exchange of metabolic signals between the periphery and the brain that are relevant for the regulation of whole-body energy homeostasis.
© 2022. Springer Nature Limited.
Conflict of interest statement
The authors have no competing interests to declare.
Figures


References
-
- Garcia-Caceres C, et al. Role of astrocytes, microglia, and tanycytes in brain control of systemic metabolism. Nat Neurosci. 2019;22:7–14. - PubMed
-
- Chowen JA, Frago LM, Fernandez-Alfonso MS. Physiological and pathophysiological roles of hypothalamic astrocytes in metabolism. J Neuroendocrinol. 2019;31:e12671. - PubMed
-
- Clasadonte J, Prevot V. The special relationship: glia-neuron interactions in the neuroendocrine hypothalamus. Nat Rev Endocrinol. 2018;14:25–44. - PubMed
-
- Al Massadi O, Lopez M, Tschop M, Dieguez C, Nogueiras R. Current Understanding of the Hypothalamic Ghrelin Pathways Inducing Appetite and Adiposity. Trends Neurosci. 2017;40:167–180. - PubMed
-
- Friedman JM. Leptin and the endocrine control of energy balance. Nat Metab. 2019;1:754–764. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

