Sample size recalculation based on the prevalence in a randomized test-treatment study
- PMID: 35879675
- PMCID: PMC9317230
- DOI: 10.1186/s12874-022-01678-7
Sample size recalculation based on the prevalence in a randomized test-treatment study
Abstract
Background: Randomized test-treatment studies aim to evaluate the clinical utility of diagnostic tests by providing evidence on their impact on patient health. However, the sample size calculation is affected by several factors involved in the test-treatment pathway, including the prevalence of the disease. Sample size planning is exposed to strong uncertainties in terms of the necessary assumptions, which have to be compensated for accordingly by adjusting prospectively determined study parameters during the course of the study.
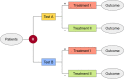
Method: An adaptive design with a blinded sample size recalculation in a randomized test-treatment study based on the prevalence is proposed and evaluated by a simulation study. The results of the adaptive design are compared to those of the fixed design.
Results: The adaptive design achieves the desired theoretical power, under the assumption that all other nuisance parameters have been specified correctly, while wrong assumptions regarding the prevalence may lead to an over- or underpowered study in the fixed design. The empirical type I error rate is sufficiently controlled in the adaptive design as well as in the fixed design.
Conclusion: The consideration of a blinded recalculation of the sample size already during the planning of the study may be advisable in order to increase the possibility of success as well as an enhanced process of the study. However, the application of the method is subject to a number of limitations associated with the study design in terms of feasibility, sample sizes needed to be achieved, and fulfillment of necessary prerequisites.
Keywords: Adaptive design; Prevalence; Sample size recalculation; Sensitivity; Specificity.
© 2022. The Author(s).
Conflict of interest statement
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures

Similar articles
-
Sample size recalculation based on the overall success rate in a randomized test-treatment trial with restricting randomization to discordant pairs.BMC Med Res Methodol. 2025 Mar 18;25(1):74. doi: 10.1186/s12874-024-02410-3. BMC Med Res Methodol. 2025. PMID: 40102729 Free PMC article.
-
Blinded sample size re-estimation in a comparative diagnostic accuracy study.BMC Med Res Methodol. 2022 Apr 19;22(1):115. doi: 10.1186/s12874-022-01564-2. BMC Med Res Methodol. 2022. PMID: 35439947 Free PMC article.
-
Sample size recalculation in multicenter randomized controlled clinical trials based on noncomparative data.Biom J. 2020 Sep;62(5):1284-1299. doi: 10.1002/bimj.201900138. Epub 2020 Mar 4. Biom J. 2020. PMID: 32128868
-
Sample size recalculation in internal pilot study designs: a review.Biom J. 2006 Aug;48(4):537-55. doi: 10.1002/bimj.200510238. Biom J. 2006. PMID: 16972704 Review.
-
Subgroup analyses in randomised controlled trials: quantifying the risks of false-positives and false-negatives.Health Technol Assess. 2001;5(33):1-56. doi: 10.3310/hta5330. Health Technol Assess. 2001. PMID: 11701102 Review.
Cited by
-
A diagnostic phase III/IV seamless design to investigate the diagnostic accuracy and clinical effectiveness using the example of HEDOS and HEDOS II.Stat Methods Med Res. 2024 Mar;33(3):433-448. doi: 10.1177/09622802241227951. Epub 2024 Feb 7. Stat Methods Med Res. 2024. PMID: 38327081 Free PMC article. Clinical Trial.
-
A unified framework for diagnostic test development and evaluation during outbreaks of emerging infections.Commun Med (Lond). 2024 Dec 10;4(1):263. doi: 10.1038/s43856-024-00691-9. Commun Med (Lond). 2024. PMID: 39658579 Free PMC article. Review.
-
Point-of-care tests in the emergency medical services: a scoping review.Scand J Trauma Resusc Emerg Med. 2025 Feb 3;33(1):18. doi: 10.1186/s13049-025-01329-y. Scand J Trauma Resusc Emerg Med. 2025. PMID: 39901298 Free PMC article.
-
Sample size recalculation based on the overall success rate in a randomized test-treatment trial with restricting randomization to discordant pairs.BMC Med Res Methodol. 2025 Mar 18;25(1):74. doi: 10.1186/s12874-024-02410-3. BMC Med Res Methodol. 2025. PMID: 40102729 Free PMC article.
References
-
- Schünemann AHJ, Oxman AD, Brozek J, et al. GRADE: grading of quality of evidence and strength of recommendations for diagnostic tests and strategies. Br Med J. 2008;336(May). 10.1136/bmj.39500.677199.AE. - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

