Short incision versus minimally invasive surgery with tool-kit for carpal tunnel syndrome release: a prospective randomized control trial to evaluate the anterior wrist pain and time to return to work or activities
- PMID: 35879713
- PMCID: PMC9316708
- DOI: 10.1186/s12891-022-05663-5
Short incision versus minimally invasive surgery with tool-kit for carpal tunnel syndrome release: a prospective randomized control trial to evaluate the anterior wrist pain and time to return to work or activities
Abstract
Trial design: The prospective randomized controlled trial.
Background: This study compares outcomes in terms of early postoperative anterior wrist pain and time to return to work or activities of daily living of patients who underwent carpal tunnel syndrome (CTS) release with short incision and those who had minimally invasive surgery (MIS) with CTS kits.
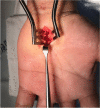
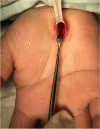
Methods: A total of 24 patients diagnosed with primary CTS confirmed with electrodiagnosis at an academic university hospital were randomly assigned into one of two groups of 12 patients each: a short incision group and an MIS with tool-kit group using computer-generated block randomization (block of four). Sequentially numbered, opaque, sealed envelopes were used in the allocation concealment process. In the short incision group, skin was incised longitudinally from Kaplan's line to the area distal to transverse wrist crease (2.5-4.0 cm) while in the tool-kit group, an incision of less than 2.5 cm. was made using special MIS-CTS kits. Primary outcomes evaluated include visual analogue scale (VAS) measurement of pain intensity in the anterior carpal area both while at rest and while conducting daily activities at the 2nd week postoperatively as well as the time to return to activities of daily living and work. Improvement in the Michigan hand questionnaire (MHQ) score, a secondary outcome, was also measured at the 2nd week postoperatively. Patients, allocator and outcome assessor were blinded.
Results: Demographic data, including preoperative electrodiagnostic severity and occupation, were similar in the two groups. There were no significant differences in terms of VAS of the early postoperative anterior carpal area at rest (p > 0.99), while conducting daily activities (p = 0.89) and time to return to activities of daily living (p = 0.46) and work (p = 0.24). The MHQ score improvement at the 2nd week postoperatively showed no significant difference between the groups (p = 0.95). The MIS wound length in the tool-kit group was significantly shorter than in the short incision group (1.95 vs 2.92 cm, p < 0.01).
Conclusions: There is no difference in early postoperative anterior wrist pain, time to return to work or to activities of daily living between the surgical techniques. Short incision is recommended for benefit in term of cost-effectiveness, while MIS with tool-kit could be preferred in patients who concerned in cosmetic appearance between the surgical techniques.
Trial registration: www.
Clinicaltrials: in.th (TCTR20200530003). Registered 30 May 2020.
Keywords: Anterior wrist pain; Carpal tunnel syndrome; Minimally invasive surgery; Randomized controlled trial; Short incision; Time to return to activities of daily living; Time to return to work.
© 2022. The Author(s).
Conflict of interest statement
Tool-kits (MIS-CTS kits) for minimally invasive surgery were supported by SCG Chemicals Co Ltd.
Figures
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials