Current and Future Strategies for the Diagnosis and Treatment of the Alpha-Gal Syndrome (AGS)
- PMID: 35879928
- PMCID: PMC9307871
- DOI: 10.2147/JAA.S265660
Current and Future Strategies for the Diagnosis and Treatment of the Alpha-Gal Syndrome (AGS)
Abstract
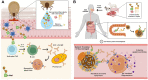
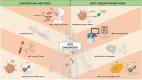
The α-Gal syndrome (AGS) is a pathognomonic immunoglobulin E (IgE)-mediated delayed anaphylaxis in foods containing the oligosaccharide galactose-α-1,3-galactose (α-Gal) such as mammalian meat or dairy products. Clinical presentation of AGS can also comprise immediate hypersensitivity due to anticancer therapy, gelatin-containing vaccines or mammalian serum-based antivenom. The IgE initial sensitization is caused by hard-bodied tick bites and symptomatic individuals typically develop delayed pruritus, urticaria, angioedema, anaphylaxis, malaise or gut-related symptoms. Due to inapparent presentation, delayed reactions and a wide variety of patients´ clinical history, the AGS diagnosis and treatment remain challenging. This review covers not only current diagnostic methods used for AGS such as the skin prick test (SPT), the oral food challenge (OFC), anti-α-Gal IgE levels measurement and the basophil activation test (BAT), but also potentially relevant next-generation diagnostic tools like the mast cell activation test (MAT), the histamine-release (HR) assay, omics technologies and model-based reasoning (MBR). Moreover, it focuses on the therapeutical medical and non-medical methods available and current research methods that are being applied in order to elucidate the molecular, physiological and immune mechanisms underlying this allergic disorder. Lastly, future treatment and preventive tools are also discussed, being of utmost importance for the identification of tick salivary molecules, with or without α-Gal modifications, that trigger IgE sensitivity as they could be the key for further vaccine development. Bearing in mind climate change, the tick-host paradigm will shift towards an increasing number of AGS cases in new regions worldwide, which will pose new challenges for clinicians in the future.
Keywords: AGS; IgE; alpha-Gal; alpha-Gal syndrome; food allergy; glycan; tick.
© 2022 Vaz-Rodrigues et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest in this work.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources

