Computational framework for single-cell spatiotemporal dynamics of optogenetic membrane recruitment
- PMID: 35880018
- PMCID: PMC9308134
- DOI: 10.1016/j.crmeth.2022.100245
Computational framework for single-cell spatiotemporal dynamics of optogenetic membrane recruitment
Abstract
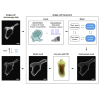
We describe a modular computational framework for analyzing cell-wide spatiotemporal signaling dynamics in single-cell microscopy experiments that accounts for the experiment-specific geometric and diffractive complexities that arise from heterogeneous cell morphologies and optical instrumentation. Inputs are unique cell geometries and protein concentrations derived from confocal stacks and spatiotemporally varying environmental stimuli. After simulating the system with a model of choice, the output is convolved with the microscope point-spread function for direct comparison with the observable image. We experimentally validate this approach in single cells with BcLOV4, an optogenetic membrane recruitment system for versatile control over cell signaling, using a three-dimensional non-linear finite element model with all parameters experimentally derived. The simulations recapitulate observed subcellular and cell-to-cell variability in BcLOV4 signaling, allowing for inter-experimental differences of cellular and instrumentation origins to be elucidated and resolved for improved interpretive robustness. This single-cell approach will enhance optogenetics and spatiotemporally resolved signaling studies.
Keywords: finite element analysis; finite element model; optogenetics; peripheral membrane protein; single cell.
© 2022 The Authors.
Conflict of interest statement
The authors declare no competing interests.
Figures






Similar articles
-
Optogenetic Control of Fibroblast Growth Factor Receptor Signaling.Methods Mol Biol. 2016;1408:345-62. doi: 10.1007/978-1-4939-3512-3_24. Methods Mol Biol. 2016. PMID: 26965135
-
Optically inducible membrane recruitment and signaling systems.Curr Opin Struct Biol. 2019 Aug;57:84-92. doi: 10.1016/j.sbi.2019.01.017. Epub 2019 Mar 16. Curr Opin Struct Biol. 2019. PMID: 30884362 Free PMC article. Review.
-
Temperature-responsive optogenetic probes of cell signaling.Nat Chem Biol. 2022 Feb;18(2):152-160. doi: 10.1038/s41589-021-00917-0. Epub 2021 Dec 22. Nat Chem Biol. 2022. PMID: 34937907 Free PMC article.
-
Optogenetic clustering and membrane translocation of the BcLOV4 photoreceptor.Proc Natl Acad Sci U S A. 2023 Aug 8;120(32):e2221615120. doi: 10.1073/pnas.2221615120. Epub 2023 Aug 1. Proc Natl Acad Sci U S A. 2023. PMID: 37527339 Free PMC article.
-
Optogenetics - Bringing light into the darkness of mammalian signal transduction.Biochim Biophys Acta Mol Cell Res. 2017 Feb;1864(2):280-292. doi: 10.1016/j.bbamcr.2016.11.009. Epub 2016 Nov 11. Biochim Biophys Acta Mol Cell Res. 2017. PMID: 27845208 Review.
References
-
- Baddeley D., Marin Z., Miguel Susano Pinto D. 2021. Python-Microscopy/Python-Microscopy: Release 20.11.25. - DOI
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

