Golexanolone, a GABAA receptor modulating steroid antagonist, restores motor coordination and cognitive function in hyperammonemic rats by dual effects on peripheral inflammation and neuroinflammation
- PMID: 35880480
- PMCID: PMC9532914
- DOI: 10.1111/cns.13926
Golexanolone, a GABAA receptor modulating steroid antagonist, restores motor coordination and cognitive function in hyperammonemic rats by dual effects on peripheral inflammation and neuroinflammation
Abstract
Aims: Hyperammonemic rats show peripheral inflammation, increased GABAergic neurotransmission and neuroinflammation in cerebellum and hippocampus which induce motor incoordination and cognitive impairment. Neuroinflammation enhances GABAergic neurotransmission in cerebellum by enhancing the TNFR1-glutaminase-GAT3 and TNFR1-CCL2-TrkB-KCC2 pathways. Golexanolone reduces GABAA receptors potentiation by allopregnanolone. This work aimed to assess if treatment of hyperammonemic rats with golexanolone reduces peripheral inflammation and neuroinflammation and restores cognitive and motor function and to analyze underlying mechanisms.
Methods: Rats were treated with golexanolone and effects on peripheral inflammation, neuroinflammation, TNFR1-glutaminase-GAT3 and TNFR1-CCL2-TrkB-KCC2 pathways, and cognitive and motor function were analyzed.
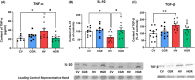
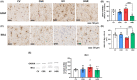
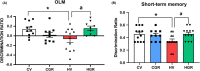
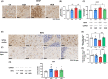
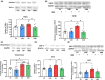
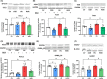
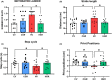
Results: Hyperammonemic rats show increased TNFα and reduced IL-10 in plasma, microglia and astrocytes activation in cerebellum and hippocampus, and impaired motor coordination and spatial and short-term memories. Treating hyperammonemic rats with golexanolone reversed changes in peripheral inflammation, microglia and astrocytes activation and restored motor coordination and spatial and short-term memory. This was associated with reversal of the hyperammonemia-enhanced activation in cerebellum of the TNFR1-glutaminase-GAT3 and TNFR1-CCL2-TrkB-KCC2 pathways.
Conclusion: Reducing GABAA receptors activation with golexanolone reduces peripheral inflammation and neuroinflammation and improves cognitive and motor function in hyperammonemic rats. The effects identified would also occur in patients with hepatic encephalopathy and, likely, in other pathologies associated with neuroinflammation.
Keywords: GR3027; inflammation; minimal hepatic encephalopathy; motor incoordination; spatial memory.
© 2022 The Authors. CNS Neuroscience & Therapeutics published by John Wiley & Sons Ltd.
Conflict of interest statement
This study was financed by Umecrine Cognition AB, which is developing GR3027/Golexanolone.
Figures
Similar articles
-
Extracellular Vesicles From Hyperammonemic Rats Induce Neuroinflammation in Cerebellum of Normal Rats: Role of Increased TNFα Content.Front Immunol. 2022 Jul 13;13:921947. doi: 10.3389/fimmu.2022.921947. eCollection 2022. Front Immunol. 2022. PMID: 35911759 Free PMC article.
-
Neuroinflammation alters GABAergic neurotransmission in hyperammonemia and hepatic encephalopathy, leading to motor incoordination. Mechanisms and therapeutic implications.Front Pharmacol. 2024 Mar 15;15:1358323. doi: 10.3389/fphar.2024.1358323. eCollection 2024. Front Pharmacol. 2024. PMID: 38560359 Free PMC article. Review.
-
Enhanced BDNF and TrkB Activation Enhance GABA Neurotransmission in Cerebellum in Hyperammonemia.Int J Mol Sci. 2022 Oct 4;23(19):11770. doi: 10.3390/ijms231911770. Int J Mol Sci. 2022. PMID: 36233065 Free PMC article.
-
Increasing extracellular cGMP in cerebellum in vivo reduces neuroinflammation, GABAergic tone and motor in-coordination in hyperammonemic rats.Brain Behav Immun. 2018 Mar;69:386-398. doi: 10.1016/j.bbi.2017.12.013. Epub 2017 Dec 27. Brain Behav Immun. 2018. PMID: 29288802
-
Peripheral inflammation induces neuroinflammation that alters neurotransmission and cognitive and motor function in hepatic encephalopathy: Underlying mechanisms and therapeutic implications.Acta Physiol (Oxf). 2019 Jun;226(2):e13270. doi: 10.1111/apha.13270. Epub 2019 Mar 22. Acta Physiol (Oxf). 2019. PMID: 30830722 Review.
Cited by
-
A transient blood IL-17 increase triggers neuroinflammation in cerebellum and motor incoordination in hyperammonemic rats.J Neuroinflammation. 2024 Nov 30;21(1):314. doi: 10.1186/s12974-024-03310-5. J Neuroinflammation. 2024. PMID: 39616376 Free PMC article.
-
Role of peripheral inflammation in minimal hepatic encephalopathy.Metab Brain Dis. 2024 Dec;39(8):1667-1677. doi: 10.1007/s11011-024-01417-5. Epub 2024 Aug 23. Metab Brain Dis. 2024. PMID: 39177864 Review.
-
Preface for the Vicente Felipo Honorary Issue of Neurochemical Research.Neurochem Res. 2024 Jun;49(6):1421-1426. doi: 10.1007/s11064-024-04139-3. Neurochem Res. 2024. PMID: 38641758 No abstract available.
-
Microbiota-gut-liver-brain axis and hepatic encephalopathy.Microbiome Res Rep. 2024 Jan 25;3(2):17. doi: 10.20517/mrr.2023.44. eCollection 2024. Microbiome Res Rep. 2024. PMID: 38841407 Free PMC article. Review.
-
The Mechanism of Hepatic Encephalopathy Induced by Thioacetamide Based on Metabolomics and Proteomics: A Preliminary Study.Int J Mol Sci. 2023 Dec 24;25(1):284. doi: 10.3390/ijms25010284. Int J Mol Sci. 2023. PMID: 38203455 Free PMC article.
References
-
- Amodio P, Montagnese S, Gatta A, Morgan MY. Characteristics of minimal hepatic encephalopathy. Metab Brain Dis. 2004;19(3–4):253‐267. - PubMed
-
- Urios A, Mangas‐Losada A, Gimenez‐Garzó C, et al. Altered postural control and stability in cirrhotic patients with minimal hepatic encephalopathy correlate with cognitive deficits. Liver Int. 2017;37(7):1013‐1022. - PubMed
-
- Mechtcheriakov S, Graziadei IW, Kugener A, et al. Motor dysfunction in patients with liver cirrhosis: impairment of handwriting. J Neurol. 2006;253(3):349‐356. - PubMed
-
- Butz M, Timmermann L, Braun M, et al. Motor impairment in liver cirrhosis without and with minimal hepatic encephalopathy. Acta Neurol Scand. 2010;122(1):27‐35. - PubMed
-
- Felipo V, Urios A, Valero P, et al. Serum nitrotyrosine and psychometric tests as indicators of impaired fitness to drive in cirrhotic patients with minimal hepatic encephalopathy. Liver Int. 2013;33(10):1478‐1489. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

