Golexanolone, a GABAA receptor modulating steroid antagonist, restores motor coordination and cognitive function in hyperammonemic rats by dual effects on peripheral inflammation and neuroinflammation
- PMID: 35880480
- PMCID: PMC9532914
- DOI: 10.1111/cns.13926
Golexanolone, a GABAA receptor modulating steroid antagonist, restores motor coordination and cognitive function in hyperammonemic rats by dual effects on peripheral inflammation and neuroinflammation
Abstract
Aims: Hyperammonemic rats show peripheral inflammation, increased GABAergic neurotransmission and neuroinflammation in cerebellum and hippocampus which induce motor incoordination and cognitive impairment. Neuroinflammation enhances GABAergic neurotransmission in cerebellum by enhancing the TNFR1-glutaminase-GAT3 and TNFR1-CCL2-TrkB-KCC2 pathways. Golexanolone reduces GABAA receptors potentiation by allopregnanolone. This work aimed to assess if treatment of hyperammonemic rats with golexanolone reduces peripheral inflammation and neuroinflammation and restores cognitive and motor function and to analyze underlying mechanisms.
Methods: Rats were treated with golexanolone and effects on peripheral inflammation, neuroinflammation, TNFR1-glutaminase-GAT3 and TNFR1-CCL2-TrkB-KCC2 pathways, and cognitive and motor function were analyzed.
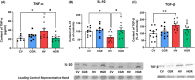
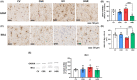
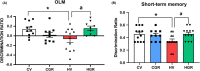
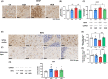
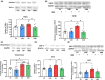
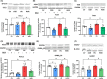
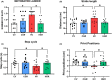
Results: Hyperammonemic rats show increased TNFα and reduced IL-10 in plasma, microglia and astrocytes activation in cerebellum and hippocampus, and impaired motor coordination and spatial and short-term memories. Treating hyperammonemic rats with golexanolone reversed changes in peripheral inflammation, microglia and astrocytes activation and restored motor coordination and spatial and short-term memory. This was associated with reversal of the hyperammonemia-enhanced activation in cerebellum of the TNFR1-glutaminase-GAT3 and TNFR1-CCL2-TrkB-KCC2 pathways.
Conclusion: Reducing GABAA receptors activation with golexanolone reduces peripheral inflammation and neuroinflammation and improves cognitive and motor function in hyperammonemic rats. The effects identified would also occur in patients with hepatic encephalopathy and, likely, in other pathologies associated with neuroinflammation.
Keywords: GR3027; inflammation; minimal hepatic encephalopathy; motor incoordination; spatial memory.
© 2022 The Authors. CNS Neuroscience & Therapeutics published by John Wiley & Sons Ltd.
Conflict of interest statement
This study was financed by Umecrine Cognition AB, which is developing GR3027/Golexanolone.
Figures
References
-
- Amodio P, Montagnese S, Gatta A, Morgan MY. Characteristics of minimal hepatic encephalopathy. Metab Brain Dis. 2004;19(3–4):253‐267. - PubMed
-
- Urios A, Mangas‐Losada A, Gimenez‐Garzó C, et al. Altered postural control and stability in cirrhotic patients with minimal hepatic encephalopathy correlate with cognitive deficits. Liver Int. 2017;37(7):1013‐1022. - PubMed
-
- Mechtcheriakov S, Graziadei IW, Kugener A, et al. Motor dysfunction in patients with liver cirrhosis: impairment of handwriting. J Neurol. 2006;253(3):349‐356. - PubMed
-
- Butz M, Timmermann L, Braun M, et al. Motor impairment in liver cirrhosis without and with minimal hepatic encephalopathy. Acta Neurol Scand. 2010;122(1):27‐35. - PubMed
-
- Felipo V, Urios A, Valero P, et al. Serum nitrotyrosine and psychometric tests as indicators of impaired fitness to drive in cirrhotic patients with minimal hepatic encephalopathy. Liver Int. 2013;33(10):1478‐1489. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

