Pathology and virulence of Edwardsiella tarda, Edwardsiella piscicida, and Edwardsiella anguillarum in channel (Ictalurus punctatus), blue (Ictalurus furcatus), and channel × blue hybrid catfish
- PMID: 35880718
- PMCID: PMC9796362
- DOI: 10.1111/jfd.13691
Pathology and virulence of Edwardsiella tarda, Edwardsiella piscicida, and Edwardsiella anguillarum in channel (Ictalurus punctatus), blue (Ictalurus furcatus), and channel × blue hybrid catfish
Abstract
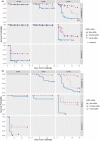
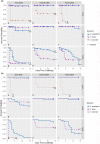
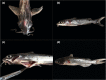
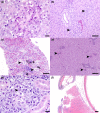
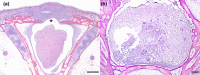
In the mid-2010s, Edwardsiella tarda was reaffiliated into three discrete taxa (E. anguillarum, E. piscicida, and E. tarda), obscuring previous descriptions of E. tarda-induced pathology in fish. To clarify ambiguity regarding the pathology of E. tarda, E. piscicida, and E. anguillarum infections in US farm-raised catfish, channel catfish (Ictalurus punctatus), blue catfish (I. furcatus), and channel × blue catfish hybrids were challenged with comparable doses of each bacterium. The most severe pathology and mortality occurred in fish challenged with E. piscicida, supporting previous reports of increased pathogenicity in commercially important ictalurids, while E. anguillarum and E. tarda warrant only minimal concern. Acute pathologic lesions among bacterial species were predominantly necrotizing and characteristic of gram-negative sepsis but became progressively granulomatous over time. After 100 days, survivors were exposed to the approximate median lethal doses of E. piscicida and E. ictaluri, revealing some cross-protective effects among E. piscicida, E. anguillarum, and E. ictaluri. In contrast, no fish that survived E. tarda challenge demonstrated any protection against E. piscicida or E. ictaluri. This work supports reports of increased susceptibility of channel, blue, and hybrid catfish to E. piscicida, while highlighting potential cross-protective affects among fish associated Edwardsiella spp.
Keywords: Edwardsiella anguillarum; Edwardsiella piscicida; Edwardsiella tarda; catfish; histopathology.
© 2022 The Authors. Journal of Fish Diseases published by John Wiley & Sons Ltd.
Conflict of interest statement
The author(s) declared no potential conflicts of interest.
Figures



Similar articles
-
Expanding the Spectrum of Diseases and Disease Associations Caused by Edwardsiella tarda and Related Species.Microorganisms. 2024 May 20;12(5):1031. doi: 10.3390/microorganisms12051031. Microorganisms. 2024. PMID: 38792860 Free PMC article. Review.
-
Cross-protective efficacy of a live-attenuated Edwardsiella ictaluri vaccine against heterologous Edwardsiella piscicida isolates in channel and channel × blue catfish hybrids.J Fish Dis. 2022 Jul;45(7):1001-1010. doi: 10.1111/jfd.13623. Epub 2022 Apr 25. J Fish Dis. 2022. PMID: 35467773
-
Evaluation of Edwardsiella piscicida basS and basR mutants as vaccine candidates in catfish against edwardsiellosis.J Fish Dis. 2022 Dec;45(12):1817-1829. doi: 10.1111/jfd.13703. Epub 2022 Aug 21. J Fish Dis. 2022. PMID: 36053889
-
Genetic variability of Edwardsiella piscicida isolates from Mississippi catfish aquaculture with an assessment of virulence in channel and channel × blue hybrid catfish.J Fish Dis. 2021 Nov;44(11):1725-1751. doi: 10.1111/jfd.13491. Epub 2021 Jul 12. J Fish Dis. 2021. PMID: 34251059
-
Response of cecropin transgenesis to challenge with Edwardsiella ictaluri in channel catfish Ictalurus punctatus.Fish Shellfish Immunol. 2022 Jul;126:311-317. doi: 10.1016/j.fsi.2022.05.050. Epub 2022 May 27. Fish Shellfish Immunol. 2022. PMID: 35636698 Review.
Cited by
-
Differentially Expressed Genes and Alternative Splicing Analysis Revealed the Difference in Virulence to American Eels (Anguilla rostrata) Infected by Edwardsiella anguillarum and Aeromonas hydrophila.Mar Biotechnol (NY). 2024 Nov 20;27(1):4. doi: 10.1007/s10126-024-10378-w. Mar Biotechnol (NY). 2024. PMID: 39565429
-
Current Trends in Approaches to Prevent and Control Antimicrobial Resistance in Aquatic Veterinary Medicine.Pathogens. 2025 Jul 10;14(7):681. doi: 10.3390/pathogens14070681. Pathogens. 2025. PMID: 40732727 Free PMC article. Review.
-
Virulent and Multi-drug-Resistant Edwardsiella tarda Infection in Oscar Fish: Unveiling the Threat of Mass Mortality and AMR Dissemination.Curr Microbiol. 2024 May 16;81(7):174. doi: 10.1007/s00284-024-03698-6. Curr Microbiol. 2024. PMID: 38753164
-
Expanding the Spectrum of Diseases and Disease Associations Caused by Edwardsiella tarda and Related Species.Microorganisms. 2024 May 20;12(5):1031. doi: 10.3390/microorganisms12051031. Microorganisms. 2024. PMID: 38792860 Free PMC article. Review.
-
Hematological alterations and bacterial abundance of Edwardsiella tarda in catfish (Clarias Sp.) cohabiting with carrier silver rasbora (Rasbora argyrotaenia).Open Vet J. 2025 Apr;15(4):1654-1662. doi: 10.5455/OVJ.2025.v15.i4.17. Epub 2025 Apr 30. Open Vet J. 2025. PMID: 40453842 Free PMC article.
References
-
- Aarattuthodiyil, S. , Griffin, M. J. , Greenway, T. E. , Khoo, L. H. , Byars, T. S. , Lewis, M. , Steadman, J. , & Wise, D. J. (2020). An orally delivered, live‐attenuated Edwardsiella ictaluri vaccine efficiently protects channel catfish fingerlings against multiple Edwardsiella ictaluri field isolates. Journal of the World Aquaculture Society, 51(6), 1354–1372. 10.1111/jwas.12693 - DOI
MeSH terms
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

