Lung evolution in vertebrates and the water-to-land transition
- PMID: 35880746
- PMCID: PMC9323002
- DOI: 10.7554/eLife.77156
Lung evolution in vertebrates and the water-to-land transition
Abstract
A crucial evolutionary change in vertebrate history was the Palaeozoic (Devonian 419-359 million years ago) water-to-land transition, allowed by key morphological and physiological modifications including the acquisition of lungs. Nonetheless, the origin and early evolution of vertebrate lungs remain highly controversial, particularly whether the ancestral state was paired or unpaired. Due to the rarity of fossil soft tissue preservation, lung evolution can only be traced based on the extant phylogenetic bracket. Here we investigate, for the first time, lung morphology in extensive developmental series of key living lunged osteichthyans using synchrotron x-ray microtomography and histology. Our results shed light on the primitive state of vertebrate lungs as unpaired, evolving to be truly paired in the lineage towards the tetrapods. The water-to-land transition confronted profound physiological challenges and paired lungs were decisive for increasing the surface area and the pulmonary compliance and volume, especially during the air-breathing on land.
Keywords: actinopterygii; developmental biology; evolutionary biology; osteichthyes; sarcopterygii; tetrapod.
Plain language summary
All life on Earth started out under water. However, around 400 million years ago some vertebrates, such as fish, started developing limbs and other characteristics that allowed them to explore life on land. One of the most pivotal features to evolve was the lungs, which gave vertebrates the ability to breathe above water. Most land-living vertebrates, including humans, have two lungs which sit on either side of their chest. The lungs extract oxygen from the atmosphere and transfer it to the bloodstream in exchange for carbon dioxide which then gets exhaled out in to the atmosphere. How this important organ first evolved is a hotly debated topic. This is largely because lung tissue does not preserve well in fossils, making it difficult to trace how the lungs of vertebrates changed over the course of evolution. To overcome this barrier, Cupello et al. compared the lungs of living species which are crucial to understand the early stages of the water-to-land transition. This included four species of lunged bony fish which breathe air at the water surface, and a four-legged salamander that lives on land. Cupello et al. used a range of techniques to examine how the lungs of the bony fish and salamander changed shape during development. The results suggested that the lungs of vertebrates started out as a single organ, which became truly paired later in evolution once vertebrates started developing limbs. This anatomical shift increased the surface area available for exchanging oxygen and carbon dioxide so that vertebrates could breathe more easily on land. These findings provide new insights in to how the lung evolved into the paired structure found in most vertebrates alive today. It likely that this transition allowed vertebrates to fully adapt to breathing above water, which may explain why this event only happened once over the course of evolution.
© 2022, Cupello et al.
Conflict of interest statement
CC, TH, NT, YY, PG, SI, RM, TS, AK, MH, KU, MO, PB No competing interests declared
Figures


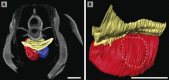

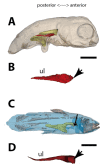

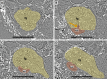





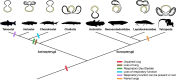
References
-
- Amemiya CT, Alföldi J, Lee AP, Fan S, Philippe H, Maccallum I, Braasch I, Manousaki T, Schneider I, Rohner N, Organ C, Chalopin D, Smith JJ, Robinson M, Dorrington RA, Gerdol M, Aken B, Biscotti MA, Barucca M, Baurain D, Berlin AM, Blatch GL, Buonocore F, Burmester T, Campbell MS, Canapa A, Cannon JP, Christoffels A, De Moro G, Edkins AL, Fan L, Fausto AM, Feiner N, Forconi M, Gamieldien J, Gnerre S, Gnirke A, Goldstone JV, Haerty W, Hahn ME, Hesse U, Hoffmann S, Johnson J, Karchner SI, Kuraku S, Lara M, Levin JZ, Litman GW, Mauceli E, Miyake T, Mueller MG, Nelson DR, Nitsche A, Olmo E, Ota T, Pallavicini A, Panji S, Picone B, Ponting CP, Prohaska SJ, Przybylski D, Saha NR, Ravi V, Ribeiro FJ, Sauka-Spengler T, Scapigliati G, Searle SMJ, Sharpe T, Simakov O, Stadler PF, Stegeman JJ, Sumiyama K, Tabbaa D, Tafer H, Turner-Maier J, van Heusden P, White S, Williams L, Yandell M, Brinkmann H, Volff JN, Tabin CJ, Shubin N, Schartl M, Jaffe DB, Postlethwait JH, Venkatesh B, Di Palma F, Lander ES, Meyer A, Lindblad-Toh K. The African coelacanth genome provides insights into tetrapod evolution. Nature. 2013;496:311–316. doi: 10.1038/nature12027. - DOI - PMC - PubMed
-
- Arsenault M, Desbiens S, Janvier P, Kerr J. In: Recent Advances in the Origin and Early Radiation of Vertebrates. Arratia G, Wilson MVH, Cloutier R, editors. Munich, Germany: Verlag Dr. Friedrich Pfeil; 2004. New data on the soft tissues and external morphology of the antiarch Bothriolepis canadensis (Whiteaves, 1880) from the Upper Devonian of Miguasha, Québec; pp. 439–454.
-
- Bartsch P, Gemballa S, Piotrowski T. The embryonic and larval development of Polypterus senegalus cuvier, 1829: its staging with reference to external and skeletal features, behaviour and locomotory habits. Acta Zoologica. 1997;78:309–328. doi: 10.1111/j.1463-6395.1997.tb01014.x. - DOI
-
- Béchard I, Arsenault F, Cloutier R, Kerr J. The Devonian placoderm fish Bothriolepis canadensis revisited with three-dimensional digital imagery. Palaeontologia Electronica. 2014;17:1–19. doi: 10.26879/417. - DOI
-
- Bi X, Wang K, Yang L, Pan H, Jiang H, Wei Q, Fang M, Yu H, Zhu C, Cai Y, He Y, Gan X, Zeng H, Yu D, Zhu Y, Jiang H, Qiu Q, Yang H, Zhang YE, Wang W, Zhu M, He S, Zhang G. Tracing the genetic footprints of vertebrate landing in non-teleost ray-finned fishes. Cell. 2021;184:1377–1391. doi: 10.1016/j.cell.2021.01.046. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources

